FRET analysis reveals distinct conformations of IN tetramers in the presence of viral DNA or LEDGF/p75
- PMID: 21771857
- PMCID: PMC3203615
- DOI: 10.1093/nar/gkr581
FRET analysis reveals distinct conformations of IN tetramers in the presence of viral DNA or LEDGF/p75
Abstract
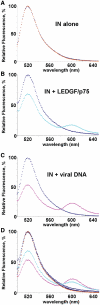
A tetramer of HIV-1 integrase (IN) stably associates with the viral DNA ends to form a fully functional concerted integration intermediate. LEDGF/p75, a key cellular binding partner of the lentiviral enzyme, also stabilizes a tetrameric form of IN. However, functional assays have indicated the importance of the order of viral DNA and LEDGF/p75 addition to IN for productive concerted integration. Here, we employed Förster Resonance Energy Transfer (FRET) to monitor assembly of individual IN subunits into tetramers in the presence of viral DNA and LEDGF/p75. The IN-viral DNA and IN-LEDGF/p75 complexes yielded significantly different FRET values suggesting two distinct IN conformations in these complexes. Furthermore, the order of addition experiments indicated that FRET for the preformed IN-viral DNA complex remained unchanged upon its subsequent binding to LEDGF/p75, whereas pre-incubation of LEDGF/p75 and IN followed by addition of viral DNA yielded FRET very similar to the IN-LEDGF/p75 complex. These findings provide new insights into the structural organization of IN subunits in functional concerted integration intermediates and suggest that differential multimerization of IN in the presence of various ligands could be exploited as a plausible therapeutic target for development of allosteric inhibitors.
Figures





References
-
- Brown PO. Integration. In: Coffin JM, Hughes SH, Varmus HE, editors. Retroviruses. Plainview, NY: Cold Spring Harbor Laboratory; 1997. pp. 161–204. - PubMed
-
- Llano M, Saenz DT, Meehan A, Wongthida P, Peretz M, Walker WH, Teo W, Poeschla EM. An essential role for LEDGF/p75 in HIV integration. Science. 2006;314:461–464. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

