Absence of the glucagon-like peptide-1 receptor does not affect the metabolic phenotype of mice with liver-specific G(s)α deficiency
- PMID: 21771891
- PMCID: PMC3159780
- DOI: 10.1210/en.2011-0012
Absence of the glucagon-like peptide-1 receptor does not affect the metabolic phenotype of mice with liver-specific G(s)α deficiency
Abstract
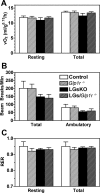
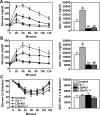
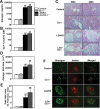
The stimulatory G protein α-subunit (G(s)α) couples hormone and other receptors to the generation of intracellular cAMP. We previously showed that mice with liver-specific G(s)α deficiency [liver-specific G(s)α knockout (LGsKO) mice] had reduced adiposity and improved glucose tolerance associated with increased glucose-stimulated insulin secretion, pancreatic islet hyperplasia, and very high serum glucagon and glucagon-like peptide 1 (GLP-1) levels. Because GLP-1 is known to stimulate insulin secretion and to have effects on energy balance, we mated LGsKO mice with germline GLP-1 receptor (GLP-1R) knockout mice (Glp1r(-/-)) and compared LGsKO to double-knockout (LGs/Glp1r(-/-)) mice to determine the contribution of excess GLP-1R signaling to the LGsKO phenotype. Loss of the GLP-1R failed to reverse most of the metabolic features of LGsKO mice, including reduced fat mass, increased glucose tolerance, and second-phase glucose-stimulated insulin secretion, islet cell hyperplasia, and very high glucagon and GLP-1 levels. However, loss of GLP-1R impaired first-phase insulin secretion in mice with or without liver-specific G(s)α deficiency. Thus, excess GLP-1 action (or at least through GLP-1R) does not contribute to the LGsKO metabolic phenotype, and other unknown factors involved in the cross talk between the liver G(s)α/cAMP pathway and pancreatic islet function need to be further elucidated.
Figures


References
-
- Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, Spiegelman B, Montminy M. 2001. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 413:179–183 - PubMed
-
- Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM. 2001. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 413:131–138 - PubMed
-
- Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, Spiegelman BM. 2003. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1α interaction. Nature 423:550–555 - PubMed
-
- Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, Hedrick S, Xu W, Boussouar F, Brindle P, Takemori H, Montminy M. 2005. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature 437:1109–1111 - PubMed
-
- Dentin R, Liu Y, Koo SH, Hedrick S, Vargas T, Heredia J, Yates J, 3rd, Montminy M. 2007. Insulin modulates gluconeogenesis by inhibition of the coactivator TORC2. Nature 449:366–369 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

