Crystal structure of the β2 adrenergic receptor-Gs protein complex
- PMID: 21772288
- PMCID: PMC3184188
- DOI: 10.1038/nature10361
Crystal structure of the β2 adrenergic receptor-Gs protein complex
Abstract
G protein-coupled receptors (GPCRs) are responsible for the majority of cellular responses to hormones and neurotransmitters as well as the senses of sight, olfaction and taste. The paradigm of GPCR signalling is the activation of a heterotrimeric GTP binding protein (G protein) by an agonist-occupied receptor. The β(2) adrenergic receptor (β(2)AR) activation of Gs, the stimulatory G protein for adenylyl cyclase, has long been a model system for GPCR signalling. Here we present the crystal structure of the active state ternary complex composed of agonist-occupied monomeric β(2)AR and nucleotide-free Gs heterotrimer. The principal interactions between the β(2)AR and Gs involve the amino- and carboxy-terminal α-helices of Gs, with conformational changes propagating to the nucleotide-binding pocket. The largest conformational changes in the β(2)AR include a 14 Å outward movement at the cytoplasmic end of transmembrane segment 6 (TM6) and an α-helical extension of the cytoplasmic end of TM5. The most surprising observation is a major displacement of the α-helical domain of Gαs relative to the Ras-like GTPase domain. This crystal structure represents the first high-resolution view of transmembrane signalling by a GPCR.
© 2011 Macmillan Publishers Limited. All rights reserved
Figures
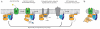


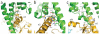
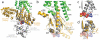

Comment in
-
Structural biology: snapshot of a signalling complex.Nature. 2011 Sep 28;477(7366):540-1. doi: 10.1038/477540a. Nature. 2011. PMID: 21956322 No abstract available.
References
-
- Dixon RA, et al. Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature. 1986;321:75–79. doi:10.1038/321075a0. - PubMed
-
- Rasmussen SG, et al. Crystal structure of the human beta2 adrenergic G-protein-coupled receptor. Nature. 2007;450:383–387. doi:nature06325 [pii] 10.1038/nature06325. - PubMed
-
- Rosenbaum DM, et al. GPCR engineering yields high-resolution structural insights into beta2-adrenergic receptor function. Science. 2007;318:1266–1273. doi:1150609 [pii] 10.1126/science.1150609. - PubMed
-
- Lefkowitz RJ. Seven transmembrane receptors: something old, something new. Acta Physiol (Oxf) 2007;190:9–19. doi:APS1693 [pii] 10.1111/j.1365-201X.2007.01693.x. - PubMed
-
- Brandt DR, Asano T, Pedersen SE, Ross EM. Reconstitution of catecholamine-stimulated guanosinetriphosphatase activity. Biochemistry. 1983;22:4357–4362. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- R01 GM075915/GM/NIGMS NIH HHS/United States
- P50GM073210/GM/NIGMS NIH HHS/United States
- P60DK-20572/DK/NIDDK NIH HHS/United States
- T32-GM008270/GM/NIGMS NIH HHS/United States
- GM75915/GM/NIGMS NIH HHS/United States
- P60 DK020572/DK/NIDDK NIH HHS/United States
- U54GM094599/GM/NIGMS NIH HHS/United States
- P50 GM073210/GM/NIGMS NIH HHS/United States
- R01 NS028471/NS/NINDS NIH HHS/United States
- NS028471/NS/NINDS NIH HHS/United States
- GM083118/GM/NIGMS NIH HHS/United States
- U54 GM094599/GM/NIGMS NIH HHS/United States
- R01 GM083118/GM/NIGMS NIH HHS/United States
- P01 GM075913/GM/NIGMS NIH HHS/United States
- P01 GM75913/GM/NIGMS NIH HHS/United States
- R01 GM056169/GM/NIGMS NIH HHS/United States
- R37 NS028471/NS/NINDS NIH HHS/United States
- GM56169/GM/NIGMS NIH HHS/United States
- R01 GM068603/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

