A Molecular Toolbox for Rapid Generation of Viral Vectors to Up- or Down-Regulate Neuronal Gene Expression in vivo
- PMID: 21772812
- PMCID: PMC3131526
- DOI: 10.3389/fnmol.2011.00008
A Molecular Toolbox for Rapid Generation of Viral Vectors to Up- or Down-Regulate Neuronal Gene Expression in vivo
Abstract
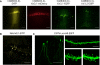
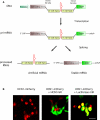
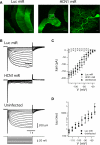
We introduce a molecular toolbox for manipulation of neuronal gene expression in vivo. The toolbox includes promoters, ion channels, optogenetic tools, fluorescent proteins, and intronic artificial microRNAs. The components are easily assembled into adeno-associated virus (AAV) or lentivirus vectors using recombination cloning. We demonstrate assembly of toolbox components into lentivirus and AAV vectors and use these vectors for in vivo expression of inwardly rectifying potassium channels (Kir2.1, Kir3.1, and Kir3.2) and an artificial microRNA targeted against the ion channel HCN1 (HCN1 miRNA). We show that AAV assembled to express HCN1 miRNA produces efficacious and specific in vivo knockdown of HCN1 channels. Comparison of in vivo viral transduction using HCN1 miRNA with mice containing a germ line deletion of HCN1 reveals similar physiological phenotypes in cerebellar Purkinje cells. The easy assembly and re-usability of the toolbox components, together with the ability to up- or down-regulate neuronal gene expression in vivo, may be useful for applications in many areas of neuroscience.
Keywords: AAV; RNAi; cerebellum; hippocampus; intronic miRNA; ion channel; lentivirus; virus.
Figures




References
-
- Abordo-Adesida E., Follenzi A., Barcia C., Sciascia S., Castro M. G., Naldini L., Lowenstein P. R. (2005). Stability of lentiviral vector-mediated transgene expression in the brain in the presence of systemic antivector immune responses. Hum. Gene Ther. 16, 741–751 10.1089/hum.2005.16.741 - DOI - PMC - PubMed
-
- Birmingham A., Anderson E. M., Reynolds A., Ilsley-Tyree D., Leake D., Fedorov Y., Baskerville S., Maksimova E., Robinson K., Karpilow J., Marshall W. S., Khvorova A. (2006). 3′ UTR seed matches, but not overall identity, are associated with RNAi off-targets. Nat. Methods 3, 199–204 10.1038/nmeth0606-487a - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

