Multimodality imaging of tumor response to doxil
- PMID: 21772927
- PMCID: PMC3139195
- DOI: 10.7150/thno/v01p0302
Multimodality imaging of tumor response to doxil
Abstract
Purpose: Early assessment of tumor responses to chemotherapy could enhance treatment outcomes by ensuring that, from the beginning, treatments meet the individualized needs of patients. In this study, we applied multiple modality molecular imaging techniques to pre-clinical monitoring of early tumor responses to Doxil, focusing on imaging of apoptosis.
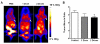
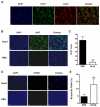
Methods: Mice bearing UM-SCC-22B human head and neck squamous cancer tumors received either PBS or 1 to 2 doses of Doxil® (doxorubicin HCl liposome injection) (10 mg/kg/dose). Bioluminescence signals from an apoptosis-responsive reporter gene were captured for apoptosis evaluation. Tumor metabolism and proliferation were assessed by( 18)F-FDG and 3'-(18)F-fluoro-3'-deoxythymidine ((18)F-FLT) positron emission tomography. Diffusion-weighted magnetic resonance imaging (DW-MRI) was performed to calculate averaged apparent diffusion coefficients (ADCs) for the whole tumor volume. After imaging, tumor samples were collected for histological evaluation, including terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL), anti-CD31, and Ki-67 immunostaining.
Results: Two doses of Doxil significantly inhibited tumor growth. Bioluminescence imaging (BLI) indicated apoptosis of tumor cells after just 1 dose of Doxil treatment, before apparent tumor shrinkage. (18)F-FDG and (18)F-FLT PET imaging identified decreased tumor metabolism and proliferation at later time points than those at which BLI indicated apoptosis. MRI measurements of ADC altered in response to Doxil, but only after tumors were treated with 2 doses. Decreased tumor proliferation and increased apoptotic cells were confirmed by changes of Ki-67 index and apoptotic ratio.
Conclusion: Our study of tumor responses to different doses of Doxil demonstrated that it is essential to combine apoptosis imaging strategies with imaging of other critical biological or pathological pathways, such as metabolism and proliferation, to improve clinical decision making in apoptosis-related diseases and interventions.
Keywords: Apoptosis; Cancer Therapy; Doxil; Multimodality Imaging; Response Monitoring.
Conflict of interest statement
Conflict of Interest: The authors have declared that no conflict of interest exists.
Figures



References
-
- Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–8. - PubMed
-
- Young RC, Ozols RF, Myers CE. The anthracycline antineoplastic drugs. N Engl J Med. 1981;305:139–53. - PubMed
-
- Tewey KM, Rowe TC, Yang L, Halligan BD, Liu LF. Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science. 1984;226:466–8. - PubMed
-
- Booser DJ, Hortobagyi GN. Anthracycline antibiotics in cancer therapy. Focus on drug resistance. Drugs. 1994;47:223–58. - PubMed
-
- Keizer HG, Pinedo HM, Schuurhuis GJ, Joenje H. Doxorubicin (adriamycin): a critical review of free radical-dependent mechanisms of cytotoxicity. Pharmacol Ther. 1990;47:219–31. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

