Prescription drug use during pregnancy in developed countries: a systematic review
- PMID: 21774029
- PMCID: PMC3423446
- DOI: 10.1002/pds.2184
Prescription drug use during pregnancy in developed countries: a systematic review
Abstract
Purpose: To review the literature describing patterns of outpatient prescription drug use during pregnancy by therapeutic category, potential for fetal harm, and overall.
Methods: We conducted a systematic review of peer-reviewed literature published from 1989 to 2010. We included studies evaluating individual-level exposures to prescription medicines during pregnancy. We selected only studies conducted in developed (Organization of Economic Co-operation and Development) countries and published in English.
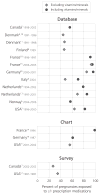
Results: Published drug utilization studies reveal wide variation in estimates of overall prescription drug use in pregnancy (27-93% of pregnant women filling at least one prescription excluding vitamins and minerals). Among studies of similar design, estimates were lowest in Northern European countries (44-47%) and highest in France (93%) and Germany (85%). Measured rates of use of contraindicated medicines in pregnancy ranged from 0.9% (Denmark, 1991-1996) to 4.6% (USA, 1996-2000). The use of medicines with positive evidence of risk ranged from 2.0% (Italy, 2004) to 59.3% (France, 1996).
Conclusion: Avoidable inconsistencies in study design and reporting attenuate conclusions that can be drawn from the literature on antenatal drug utilization. Nevertheless, the body of published research shows that antenatal prescription drug use is common, with many studies finding that a majority of women use one or more prescription medicine during pregnancy. Similarly, studies consistently report the use of drugs recognized as having potential risks in pregnancy. Given this widespread use, it is particularly important to develop standards for calculating and reporting antenatal exposures to improve the value of future research in this area.
Copyright © 2011 John Wiley & Sons, Ltd.
Conflict of interest statement
The authors have no conflicts of interest to declare.
References
-
- Bonati M, Bortolus R, Marchetti F, Romero M, Tognoni G. Drug use in pregnancy: an overview of epidemiological (drug utilization) studies. European Journal of Clinical Pharmacology. 1990;38(4):325–8. - PubMed
-
- Organization for Economic Co-operation and Development. Members and partners. 2010 Available from: http://www.oecd.org/pages/0,3417,en_36734052_36761800_1_1_1_1_1,00.html.
-
- Andrade S, Gurwitz J, Davis R, Chan K, Finkelstein J, Fortman K, et al. Prescription drug use in pregnancy. American Journal of Obstetrics & Gynecology. 2004;191(2):398–407. - PubMed
-
- Bakker M, Jentink J, Vroom F, Van Den Berg P, De Walle H, De Jong-Van Den Berg L. Drug prescription patterns before, during and after pregnancy for chronic, occasional and pregnancy-related drugs in the Netherlands. BJOG. 2006;113(5):559–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources