The isoenzyme of glutaminyl cyclase is an important regulator of monocyte infiltration under inflammatory conditions
- PMID: 21774078
- PMCID: PMC3377097
- DOI: 10.1002/emmm.201100158
The isoenzyme of glutaminyl cyclase is an important regulator of monocyte infiltration under inflammatory conditions
Abstract
Acute and chronic inflammatory disorders are characterized by detrimental cytokine and chemokine expression. Frequently, the chemotactic activity of cytokines depends on a modified N-terminus of the polypeptide. Among those, the N-terminus of monocyte chemoattractant protein 1 (CCL2 and MCP-1) is modified to a pyroglutamate (pE-) residue protecting against degradation in vivo. Here, we show that the N-terminal pE-formation depends on glutaminyl cyclase activity. The pE-residue increases stability against N-terminal degradation by aminopeptidases and improves receptor activation and signal transduction in vitro. Genetic ablation of the glutaminyl cyclase iso-enzymes QC (QPCT) or isoQC (QPCTL) revealed a major role of isoQC for pE(1) -CCL2 formation and monocyte infiltration. Consistently, administration of QC-inhibitors in inflammatory models, such as thioglycollate-induced peritonitis reduced monocyte infiltration. The pharmacologic efficacy of QC/isoQC-inhibition was assessed in accelerated atherosclerosis in ApoE3*Leiden mice, showing attenuated atherosclerotic pathology following chronic oral treatment. Current strategies targeting CCL2 are mainly based on antibodies or spiegelmers. The application of small, orally available inhibitors of glutaminyl cyclases represents an alternative therapeutic strategy to treat CCL2-driven disorders such as atherosclerosis/restenosis and fibrosis.
Copyright © 2011 EMBO Molecular Medicine.
Figures

Immature forms of human MCPs, e.g. Q1-CCL2 display cleavage sites for ApP (1) and DP4 (2). CCL2 can also be processed by MMP-1 (3). All human MCPs are putative substrates of QC/isoQC and they contain in their mature state an N-terminal pE-residue and are protected against aminopeptidase cleavage but not against MMP-1 cleavage.
N-terminal degradation of human Q1-CCL2 and pE1-CCL2 in human plasma monitored using MALDI-TOF mass spectrometry.
Internalization of CCR2 from the surface of THP-1 monocytes triggered by CCL2 variants and determined by FACS analysis. (***p < 0.001 and *p < 0.05 vs. control; ###p < 0.001 and ##p < 0.01 vs. D3, ++p < 0.01 vs. Q1, one-way ANOVA followed by Tukey post hoc test, n = 3–8, mean ± SEM).
Dependence of THP-1 monocyte migration on the concentration of CCL2(Q1-76) and CCL2(pE1-76), assessed using a chamber assay and quantification of cells by FACS analysis (***p < 0.001, **p < 0.01, Q1-CCL2 vs. pE1-CCL2, two-way ANOVA followed by Bonferroni post-test, n = 3–7, mean ± SEM).

Analysis of the ratio of total-CCL2 and pE1-CCL2 secreted from LPS-stimulated primary cells isolated from QC−/− mice compared to WT littermates (QC+/+; n = 4–5, mean ± SEM).
Analysis of the ratio of total-CCL2 and pE1-CCL2 secreted from LPS-stimulated primary cells isolated from isoQC−/− mice compared to WT littermates (isoQC+/+; ***p < 0.001 vs. isoQC+/+, Student's t-test, n = 4, mean ± SEM).
pE1-CCL2 formation in serum after peripheral injection of LPS in male QC−/− mice compared to WT littermates (QC+/+; n = 6–7, mean ± SEM).
pE1-CCL2 formation in serum after peripheral injection of LPS in male isoQC−/− mice, compared to WT littermates (QC+/+; ***p < 0.001 vs. isoQC+/+ +LPS, Student's t-test, n = 5–8, mean ± SEM).
IsoQC-deficiency leads to impaired monocyte recruitment to lungs after intranasal LPS-application in isoQC−/− compared to WT littermates (isoQC+/+; *p < 0.05 vs. isoQC+/+ +LPS, Student's t-test, n = 7–8, mean ± SEM).
Analysis of neutrophils in bronchoalveolar fluid after intranasal LPS-application in isoQC−/− compared to WT littermates (isoQC+/+; n = 7–8, mean ± SEM).

Representative FACS analysis showing the infiltrating monocyte population in QC+/+ and QC−/− mice dissected by specific staining.
Quantification of infiltrating monocytes and granulocytes in QC+/+ mice (black bars) and QC−/− mice (open bars; n = 8–13, mean ± SEM).
Lavage fluid from (B) was analyzed for total-CCL2 (black bars) and pE1-CCL2 (open bars; n.s., not significant, Student's t-test, mean ± SD).
Representative FACS analysis showing the infiltrating monocyte population in isoQC+/+ and isoQC−/− mice.
Quantification of infiltrating monocytes and granulocytes in isoQC+/+ mice (black bars) and isoQC−/− mice (open bars; ***p < 0.001 vs. isoQC+/+ Thio, Student's t-test, n = 10–14, mean ± SEM).
Lavage fluid from (E) was analyzed for total-CCL2 (black bars) and pE1-CCL2 (open bars; ***p < 0.001 vs. pE1-CCL2 from isoQC+/+ mice, Student's t-test, mean ± SD).

Analysis of total-CCL2 (black bars) and pE1-CCL2 (open bars) after application of varying doses PQ529 to LPS-stimulated primary murine glia cells isolated from C57BL/6J WT mice compared to unstimulated controls (***p < 0.001 vs. pE1-CCL2 (0 µM PQ529), ANOVA followed by Tukey post hoc test, n = 3–4, mean ± SEM).
Analysis of CCL2 gene expression in LPS-stimulated primary glia cells derived from Fig 4A(*p < 0.05, **p < 0.01 vs. PQ529 0 µM, ANOVA followed by Tukey post hoc test, n = 3–4, mean ± SEM).
Representative FACS image showing the reduction of infiltrating monocytes after application of PQ529 (30 mg/kg, i.p.).
Dose-dependent reduction of infiltrating monocytes in absence (black bars) or presence (red bars) of intraperitoneal PQ529 treatment (**p < 0.01 vs. Thio (+), ANOVA followed by Tukey post hoc test, n = 5–6, mean ± SEM, female mice).
Inhibition of monocyte infiltration after oral application of PQ50 (red bars) and PQ529 (white bar; **p < 0.01 vs. Thio (+), ANOVA followed by Tukey post hoc test, n = 5–6, mean ± SEM, female mice).
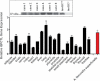

Monocyte adhesion and total adhering cells 2 days after cuff placement in absence (black bars) or presence (open bars) of PQ50 (QCI) treatment (*p < 0.05 vs. control, Student's t-test, n = 5, mean ± SD).
In addition, the CCL2-positive area was calculated in cross-sections within the media and neointima in absence (black bars) and presence (open bars) of PQ50 (QCI) treatment (**p < 0.01, ***p < 0.001 vs. control. Student's t-test, n = 5, mean ± SD).
Morphometric analysis of cuffed vessel segments shows a reduction in the degree of lumen stenosis (*p < 0.05 vs. control, Student's t-test, n = 10, mean ± SD). Inset: Example for lumen stenosis after 2 weeks (black arrows).
Neointima formation and media thickness (inset) of the cuffed vessel segments of mice sacrificed after 2 weeks, treated in absence (black bars) and presence (open bars) of QC-inhibitor PQ50 (QCI; *p < 0.05 vs. control, Student's t-test, n = 10, mean ± SD).

Comment in
-
Novel approach to inhibiting chemokine function.EMBO Mol Med. 2011 Sep;3(9):510-2. doi: 10.1002/emmm.201100161. Epub 2011 Aug 19. EMBO Mol Med. 2011. PMID: 21882341 Free PMC article. No abstract available.
References
-
- Abraham GN, Podell DN. Pyroglutamic acid. Non-metabolic formation, function in proteins and peptides, and characteristics of the enzymes effecting its removal. Mol Cell Biochem. 1981;38:181–190. - PubMed
-
- Augustin M, Sedlmeier R, Peters T, Huffstadt U, Kochmann E, Simon D, Schöniger M, Garke-Mayerthaler S, Laufs J, Mayhaus M, et al. Efficient and fast targeted production of murine models based on ENU mutagenesis. Mamm Genome. 2005;16:405–413. - PubMed
-
- Awade AC, Cleuziat P, Gonzales T, Robert-Baudouy J. Pyrrolidone carboxyl peptidase (Pcp): an enzyme that removes pyroglutamic acid (pGlu) from pGlu-peptides and pGlu-proteins. Proteins. 1994;20:34–51. - PubMed
-
- Bhatia M, Ramnath RD, Chevali L, Guglielmotti A. Treatment with bindarit, a blocker of MCP-1 synthesis, protects mice against acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1259–G1265. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

