Chiroptical detection of condensed nickel(II)-Z-DNA in the presence of the B-DNA via porphyrin exciton coupled circular dichroism
- PMID: 21774503
- PMCID: PMC3177531
- DOI: 10.1021/jp2047213
Chiroptical detection of condensed nickel(II)-Z-DNA in the presence of the B-DNA via porphyrin exciton coupled circular dichroism
Abstract
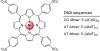
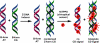
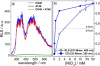
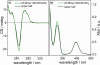
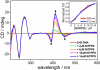
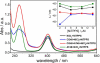
Here, we report a highly sensitive and specific chiroptical detection method of condensed left-handed Z-DNA in the presence of canonical right-handed B-DNA. The selective formation of a left-handed cytosine-guanine oligonucleotide (CG ODN) in the presence of a right-handed adenine-thymine oligonucleotide (AT ODN) was induced by millimolar concentrations of NiCl(2) and confirmed by electronic circular dichroism. The nickel(II) induced B- to Z-DNA transition of the CG ODN was accompanied by the concurrent condensation of the Ni(II)-Z-DNA, as confirmed by resonance light scattering, transmission spectroscopy, and centrifugation. The selective condensation of the CG ODN allowed its separation from the AT ODN using centrifugation. No structural changes were observed for the AT ODN upon addition of Ni(II). Anionic nickel(II) meso-tetra(4-sulfonatophenyl) porphyrin (NiTPPS) spectroscopically detected the left-handed Z-DNA in the Z-DNA/B-DNA mixture via a strong exciton coupled circular dichroism (ECCD) signal induced in the porphyrin Soret band absorption region. The bisignate ECCD signal originates from the assembly of achiral porphyrins into helical arrays by intermolecular interactions with the condensed Z-DNA scaffold. No induced CD signal was observed for the Ni(II)-B-DNA-NiTPPS complex. Hence, an unambiguous spectroscopic recognition of Ni(II) induced condensed Z-DNA in the presence of B-DNA is possible. The sensitivity of this chiroptical method was as low as 5% of the Z-DNA (4.4 μmol base pair concentration) in the presence of 95% B-DNA (80 μmol). Thus, NiTPPS is a highly sensitive probe for applications in biosensing via the CD signal amplification.
© 2011 American Chemical Society
Figures



Similar articles
-
Sulfonated Ni(II)porphyrin improves the detection of Z-DNA in condensed and non-condensed BZB DNA sequences.J Inorg Biochem. 2012 May;110:18-20. doi: 10.1016/j.jinorgbio.2012.02.001. Epub 2012 Feb 9. J Inorg Biochem. 2012. PMID: 22459169 Free PMC article.
-
Chiroptical properties of anionic and cationic porphyrins and metalloporphyrins in complex with left-handed Z-DNA and right-handed B-DNA.J Inorg Biochem. 2013 Oct;127:1-6. doi: 10.1016/j.jinorgbio.2013.05.018. Epub 2013 Jun 14. J Inorg Biochem. 2013. PMID: 23831582
-
A novel porphyrin-based molecular probe ZnTCPPSpm4 with catalytic, stabilizing and chiroptical diagnostic power towards DNA B-Z transition.J Inorg Biochem. 2017 Aug;173:141-143. doi: 10.1016/j.jinorgbio.2017.05.008. Epub 2017 May 14. J Inorg Biochem. 2017. PMID: 28525806
-
Circular dichroism and the interactions of water soluble porphyrins with DNA.Chirality. 2003 May 5;15(4):329-32. doi: 10.1002/chir.10206. Chirality. 2003. PMID: 12666240 Review.
-
Porphyrins and metalloporphyrins: versatile circular dichroic reporter groups for structural studies.Chirality. 2000 May;12(4):237-55. doi: 10.1002/(SICI)1520-636X(2000)12:4<237::AID-CHIR10>3.0.CO;2-6. Chirality. 2000. PMID: 10790194 Review.
Cited by
-
3,3'-diethylthiatricarbocyanine iodide: a highly sensitive chiroptical reporter of DNA helicity and sequence.Int J Mol Sci. 2011;12(11):8052-62. doi: 10.3390/ijms12118052. Epub 2011 Nov 16. Int J Mol Sci. 2011. PMID: 22174649 Free PMC article.
-
The Cucurbit[7]Uril-Based Supramolecular Chemistry for Reversible B/Z-DNA Transition.Adv Sci (Weinh). 2018 May 15;5(7):1800231. doi: 10.1002/advs.201800231. eCollection 2018 Jul. Adv Sci (Weinh). 2018. PMID: 30027051 Free PMC article.
-
Computational Simulation and Biophysical Study on Cerium Chloride-Induced B-to-Z Transition in (CG) n DNA.ACS Omega. 2024 Nov 16;9(47):46784-46795. doi: 10.1021/acsomega.4c04562. eCollection 2024 Nov 26. ACS Omega. 2024. PMID: 39619516 Free PMC article.
-
Chiroptical switches: applications in sensing and catalysis.Molecules. 2012 Jan 31;17(2):1247-77. doi: 10.3390/molecules17021247. Molecules. 2012. PMID: 22293845 Free PMC article. Review.
-
Porphyrins as Colorimetric and Photometric Biosensors in Modern Bioanalytical Systems.Chembiochem. 2020 Jul 1;21(13):1793-1807. doi: 10.1002/cbic.202000067. Epub 2020 Mar 30. Chembiochem. 2020. PMID: 32187831 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

