Predictors of long-term cognitive outcome in Alzheimer's disease
- PMID: 21774798
- PMCID: PMC3226278
- DOI: 10.1186/alzrt85
Predictors of long-term cognitive outcome in Alzheimer's disease
Abstract
Introduction: The objective of this study was to describe the longitudinal cognitive outcome in Alzheimer's disease (AD) and analyze factors that affect the outcome, including the impact of different cholinesterase inhibitors (ChEI).
Methods: In an open, three-year, nonrandomized, prospective, multicenter study, 843 patients were treated with donepezil, rivastigmine, or galantamine in a routine clinical setting. At baseline and every six months, patients were assessed using several rating scales, including the Mini-Mental State Examination (MMSE) and the Alzheimer's Disease Assessment Scale-cognitive subscale (ADAS-cog) and the dose of ChEI was recorded. Sociodemographic and clinical characteristics were investigated. The relationships of these predictors with longitudinal cognitive ability were analyzed using mixed-effects models.
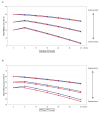
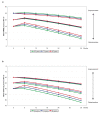
Results: Slower long-term cognitive decline was associated with a higher cognitive ability at baseline or a lower level of education. The improvement in cognitive response after six months of ChEI therapy and a more positive longitudinal outcome were related to a higher mean dose of ChEI, nonsteroidal anti-inflammatory drug (NSAID)/acetylsalicylic acid usage, male gender, older age, and absence of the apolipoprotein E (APOE) ε4 allele. More severe cognitive impairment at baseline also predicted an improved response to ChEI treatment after six months. The type of ChEI agent did not influence the short-term response or the long-term outcome.
Conclusions: In this three-year AD study performed in a routine clinical practice, the response to ChEI treatment and longitudinal cognitive outcome were better in males, older individuals, non-carriers of the APOE ε4 allele, patients treated with NSAIDs/acetylsalicylic acid, and those receiving a higher dose of ChEI, regardless of the drug agent.
Figures
Similar articles
-
Early- versus late-onset Alzheimer's disease in clinical practice: cognitive and global outcomes over 3 years.Alzheimers Res Ther. 2017 Aug 31;9(1):70. doi: 10.1186/s13195-017-0294-2. Alzheimers Res Ther. 2017. PMID: 28859660 Free PMC article.
-
Mild versus moderate stages of Alzheimer's disease: three-year outcomes in a routine clinical setting of cholinesterase inhibitor therapy.Alzheimers Res Ther. 2016 Feb 17;8:7. doi: 10.1186/s13195-016-0174-1. Alzheimers Res Ther. 2016. PMID: 26883213 Free PMC article.
-
Progression of mild Alzheimer's disease: knowledge and prediction models required for future treatment strategies.Alzheimers Res Ther. 2013 Oct 7;5(5):44. doi: 10.1186/alzrt210. eCollection 2013. Alzheimers Res Ther. 2013. PMID: 24099236 Free PMC article.
-
Donepezil and rivastigmine in the treatment of Alzheimer's disease: a best-evidence synthesis of the published data on their efficacy and cost-effectiveness.Clin Ther. 2002 Jun;24(6):862-86; discussion 837. doi: 10.1016/s0149-2918(02)80004-2. Clin Ther. 2002. PMID: 12117079 Review.
-
Effect of Apolipoprotein E ɛ4 Carrier Status on Cognitive Response to Acetylcholinesterase Inhibitors in Patients with Alzheimer's Disease: A Systematic Review and Meta-Analysis.Dement Geriatr Cogn Disord. 2018;45(5-6):335-352. doi: 10.1159/000490175. Epub 2018 Jul 24. Dement Geriatr Cogn Disord. 2018. PMID: 30041236
Cited by
-
A Systematic Review of MicroRNA Expression as Biomarker of Late-Onset Alzheimer's Disease.Mol Neurobiol. 2019 Dec;56(12):8376-8391. doi: 10.1007/s12035-019-01676-9. Epub 2019 Jun 25. Mol Neurobiol. 2019. PMID: 31240600
-
Progress in understanding variability in cognitive responses to cholinesterase inhibitor treatment.Alzheimers Res Ther. 2011 Oct 17;3(5):30. doi: 10.1186/alzrt92. Alzheimers Res Ther. 2011. PMID: 21999183 Free PMC article.
-
Sex Differences in the Preventive Effect of Cardiovascular and Metabolic Therapeutics on Dementia.Biomol Ther (Seoul). 2023 Nov 1;31(6):583-598. doi: 10.4062/biomolther.2023.115. Biomol Ther (Seoul). 2023. PMID: 37899743 Free PMC article. Review.
-
Alzheimer's disease assessment scale-cognitive 11-item progression model in mild-to-moderate Alzheimer's disease trials of bapineuzumab.Alzheimers Dement (N Y). 2015 Oct 9;1(3):157-169. doi: 10.1016/j.trci.2015.09.001. eCollection 2015 Nov. Alzheimers Dement (N Y). 2015. PMID: 29854935 Free PMC article.
-
Psychotropic and anti-dementia treatment in elderly persons with clinical signs of dementia with Lewy bodies: a cross-sectional study in 40 nursing homes in Sweden.BMC Geriatr. 2018 Feb 17;18(1):50. doi: 10.1186/s12877-018-0740-4. BMC Geriatr. 2018. PMID: 29454305 Free PMC article.
References
-
- Fratiglioni L, Grut M, Forsell Y, Viitanen M, Grafstrom M, Holmen K, Ericsson K, Backman L, Ahlbom A, Winblad B. Prevalence of Alzheimer's disease and other dementias in an elderly urban population: relationship with age, sex, and education. Neurology. 1991;41:1886–1892. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

