Sliding-induced non-uniform pre-tension governs robust and reversible adhesion: a revisit of adhesion mechanisms of geckos
- PMID: 21775325
- PMCID: PMC3243387
- DOI: 10.1098/rsif.2011.0254
Sliding-induced non-uniform pre-tension governs robust and reversible adhesion: a revisit of adhesion mechanisms of geckos
Abstract
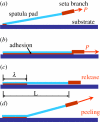
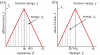
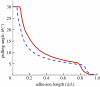
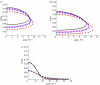
Several mechanisms have been proposed in the literature to explain the robust attachment and rapid, controllable detachment of geckos' feet on vertical walls or ceilings, yet, it is still debatable, which one is ultimately responsible for geckos' extraordinary capabilities for robust and reversible adhesion. In this paper, we re-examine some of the key movements of geckos' spatula pads and seta hairs during attachment and detachment, and propose a sequence of simple mechanical steps that would lead to the extraordinary properties of geckos observed in experiments. The central subject under study here is a linear distribution of pre-tension along the spatula pad induced by its sliding motion with respect to a surface. The resulting pre-tension, together with a control of setae's pulling force and angle, not only allows for robust and strong attachment, but also enables rapid and controllable detachment. We perform computational modelling and simulations to validate the following key steps of geckos' adhesion: (i) creation of a linear distribution of pre-tension in spatula through sliding, (ii) operation of an instability envelope controlled by setae's pulling force and angle, (iii) triggering of an adhesion instability leading to partial decohesion along the interface, and (iv) complete detachment of spatula through post-instability peeling. The present work not only reveals novel insights into the adhesion mechanism of geckos, but also develops a powerful numerical simulation approach as well as additional guidelines for bioinspired materials and devices.
Figures






Similar articles
-
Dynamic biological adhesion: mechanisms for controlling attachment during locomotion.Philos Trans R Soc Lond B Biol Sci. 2019 Oct 28;374(1784):20190199. doi: 10.1098/rstb.2019.0199. Epub 2019 Sep 9. Philos Trans R Soc Lond B Biol Sci. 2019. PMID: 31495309 Free PMC article. Review.
-
Frictional adhesion: A new angle on gecko attachment.J Exp Biol. 2006 Sep;209(Pt 18):3569-79. doi: 10.1242/jeb.02486. J Exp Biol. 2006. PMID: 16943497
-
Pre-tension generates strongly reversible adhesion of a spatula pad on substrate.J R Soc Interface. 2009 Jun 6;6(35):529-37. doi: 10.1098/rsif.2008.0322. Epub 2008 Sep 18. J R Soc Interface. 2009. PMID: 18801716 Free PMC article.
-
Adhesion and friction in gecko toe attachment and detachment.Proc Natl Acad Sci U S A. 2006 Dec 19;103(51):19320-5. doi: 10.1073/pnas.0608841103. Epub 2006 Dec 5. Proc Natl Acad Sci U S A. 2006. PMID: 17148600 Free PMC article.
-
A review of bioinspired dry adhesives: from achieving strong adhesion to realizing switchable adhesion.Bioinspir Biomim. 2024 Jul 23;19(5). doi: 10.1088/1748-3190/ad62cf. Bioinspir Biomim. 2024. PMID: 38996419 Review.
Cited by
-
Detachment of compliant films adhered to stiff substrates via van der Waals interactions: role of frictional sliding during peeling.J R Soc Interface. 2014 Aug 6;11(97):20140453. doi: 10.1098/rsif.2014.0453. J R Soc Interface. 2014. PMID: 24920120 Free PMC article.
-
Simulation of synthetic gecko arrays shearing on rough surfaces.J R Soc Interface. 2014 Apr 2;11(95):20140021. doi: 10.1098/rsif.2014.0021. Print 2014 Jun 6. J R Soc Interface. 2014. PMID: 24694893 Free PMC article.
-
Design of gecko-inspired fibrillar surfaces with strong attachment and easy-removal properties: a numerical analysis of peel-zone.J R Soc Interface. 2012 Oct 7;9(75):2424-36. doi: 10.1098/rsif.2012.0200. Epub 2012 May 9. J R Soc Interface. 2012. PMID: 22572030 Free PMC article.
-
Biomechanics of shear-sensitive adhesion in climbing animals: peeling, pre-tension and sliding-induced changes in interface strength.J R Soc Interface. 2016 Sep;13(122):20160373. doi: 10.1098/rsif.2016.0373. J R Soc Interface. 2016. PMID: 27605165 Free PMC article.
-
Dynamic biological adhesion: mechanisms for controlling attachment during locomotion.Philos Trans R Soc Lond B Biol Sci. 2019 Oct 28;374(1784):20190199. doi: 10.1098/rstb.2019.0199. Epub 2019 Sep 9. Philos Trans R Soc Lond B Biol Sci. 2019. PMID: 31495309 Free PMC article. Review.
References
-
- Autumn K., Liang Y. A., Hsieh S. T., Zesch W., Chan W. P., Kenny T. W., Fearing R., Full R. J. 2000. Adhesive force of a single gecko foot-hair. Nature 405, 681–68510.1038/35015073 (doi:10.1038/35015073) - DOI - DOI - PubMed
-
- Autumn K., Peattie A. M. 2002. Mechanisms of adhesion in geckos. Integr. Comp. Biol. 42, 1081–109010.1093/icb/42.6.1081 (doi:10.1093/icb/42.6.1081) - DOI - DOI - PubMed
-
- Jagota A., Bennison S. J. 2002. Mechanics of adhesion through a fibrillar microstructure. Integr. Comp. Biol. 42, 1140–114510.1093/icb/42.6.1140 (doi:10.1093/icb/42.6.1140) - DOI - DOI - PubMed
-
- Spolenak R., Gorb S., Gao H. J., Arzt E. 2005. Effects of contact shape on the scaling of biological attachments. Proc. R. Soc. A 461, 305–31910.1098/rspa.2004.1326 (doi:10.1098/rspa.2004.1326) - DOI - DOI
-
- Tian Y., Pesika N., Zeng H. B., Rosenberg K., Zhao B. X., McGuiggan P., Autumn K., Israelachvili J. 2006. Adhesion and friction in gecko toe attachment and detachment. Proc. Natl Acad. Sci. USA. 103, 19 320–19 32510.1073/pnas.0608841103 (doi:10.1073/pnas.0608841103) - DOI - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

