A Sox10 enhancer element common to the otic placode and neural crest is activated by tissue-specific paralogs
- PMID: 21775416
- PMCID: PMC3152925
- DOI: 10.1242/dev.057836
A Sox10 enhancer element common to the otic placode and neural crest is activated by tissue-specific paralogs
Abstract
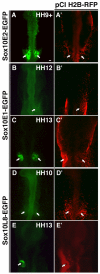
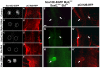
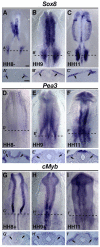
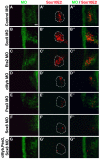
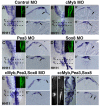
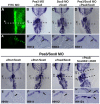
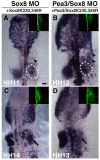
The otic placode, a specialized region of ectoderm, gives rise to components of the inner ear and shares many characteristics with the neural crest, including expression of the key transcription factor Sox10. Here, we show that in avian embryos, a highly conserved cranial neural crest enhancer, Sox10E2, also controls the onset of Sox10 expression in the otic placode. Interestingly, we show that different combinations of paralogous transcription factors (Sox8, Pea3 and cMyb versus Sox9, Ets1 and cMyb) are required to mediate Sox10E2 activity in the ear and neural crest, respectively. Mutating their binding motifs within Sox10E2 greatly reduces enhancer activity in the ear. Moreover, simultaneous knockdown of Sox8, Pea3 and cMyb eliminates not only the enhancer-driven reporter expression, but also the onset of endogenous Sox10 expression in the ear. Rescue experiments confirm that the specific combination of Myb together with Sox8 and Pea3 is responsible for the onset of Sox10 expression in the otic placode, as opposed to Myb plus Sox9 and Ets1 for neural crest Sox10 expression. Whereas SUMOylation of Sox8 is not required for the initial onset of Sox10 expression, it is necessary for later otic vesicle formation. This new role of Sox8, Pea3 and cMyb in controlling Sox10 expression via a common otic/neural crest enhancer suggests an evolutionarily conserved function for the combination of paralogous transcription factors in these tissues of distinct embryological origin.
Figures

Similar articles
-
Tissue specific regulation of the chick Sox10E1 enhancer by different Sox family members.Dev Biol. 2017 Feb 1;422(1):47-57. doi: 10.1016/j.ydbio.2016.12.004. Epub 2016 Dec 22. Dev Biol. 2017. PMID: 28012818 Free PMC article.
-
Genomic code for Sox10 activation reveals a key regulatory enhancer for cranial neural crest.Proc Natl Acad Sci U S A. 2010 Feb 23;107(8):3570-5. doi: 10.1073/pnas.0906596107. Epub 2010 Feb 5. Proc Natl Acad Sci U S A. 2010. PMID: 20139305 Free PMC article.
-
Cooperation of Sall4 and Sox8 transcription factors in the regulation of the chicken Sox3 gene during otic placode development.Dev Growth Differ. 2018 Apr;60(3):133-145. doi: 10.1111/dgd.12427. Epub 2018 Mar 8. Dev Growth Differ. 2018. PMID: 29520762
-
SoxE factors as multifunctional neural crest regulatory factors.Int J Biochem Cell Biol. 2010 Mar;42(3):441-4. doi: 10.1016/j.biocel.2009.11.014. Epub 2009 Nov 30. Int J Biochem Cell Biol. 2010. PMID: 19931641 Free PMC article. Review.
-
Conservation and diversity in the cis-regulatory networks that integrate information controlling expression of Hoxa2 in hindbrain and cranial neural crest cells in vertebrates.Dev Biol. 2002 Jun 1;246(1):45-56. doi: 10.1006/dbio.2002.0665. Dev Biol. 2002. PMID: 12027433 Review.
Cited by
-
Dynamic and differential regulation of stem cell factor FoxD3 in the neural crest is Encrypted in the genome.PLoS Genet. 2012;8(12):e1003142. doi: 10.1371/journal.pgen.1003142. Epub 2012 Dec 20. PLoS Genet. 2012. PMID: 23284303 Free PMC article.
-
SOX9 and SOX10 control fluid homeostasis in the inner ear for hearing through independent and cooperative mechanisms.Proc Natl Acad Sci U S A. 2022 Nov 16;119(46):e2122121119. doi: 10.1073/pnas.2122121119. Epub 2022 Nov 7. Proc Natl Acad Sci U S A. 2022. PMID: 36343245 Free PMC article.
-
Foxi3 is necessary for the induction of the chick otic placode in response to FGF signaling.Dev Biol. 2014 Jul 15;391(2):158-69. doi: 10.1016/j.ydbio.2014.04.014. Epub 2014 Apr 26. Dev Biol. 2014. PMID: 24780628 Free PMC article.
-
c-Myb knockdown increases the neomycin-induced damage to hair-cell-like HEI-OC1 cells in vitro.Sci Rep. 2017 Jan 23;7:41094. doi: 10.1038/srep41094. Sci Rep. 2017. PMID: 28112219 Free PMC article.
-
Lens development depends on a pair of highly conserved Sox21 regulatory elements.Dev Biol. 2012 May 1;365(1):310-8. doi: 10.1016/j.ydbio.2012.02.025. Epub 2012 Feb 24. Dev Biol. 2012. PMID: 22387845 Free PMC article.
References
-
- Bagheri-Fam S., Barrionuevo F., Dohrmann U., Gunther T., Schule R., Kemler R., Mallo M., Kanzler B., Scherer G. (2006). Long-range upstream and downstream enhancers control distinct subsets of the complex spatiotemporal Sox9 expression pattern. Dev. Biol. 291, 382-397 - PubMed
-
- Baker C. V. (2008). The evolution and elaboration of vertebrate neural crest cells. Curr. Opin. Genet. Dev. 18, 536-543 - PubMed
-
- Barrionuevo F., Naumann A., Bagheri-Fam S., Speth V., Taketo M. M., Scherer G., Neubuser A. (2008). Sox9 is required for invagination of the otic placode in mice. Dev. Biol. 317, 213-224 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

