Uncoupling protein 3 (UCP3) modulates the activity of Sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) by decreasing mitochondrial ATP production
- PMID: 21775425
- PMCID: PMC3173197
- DOI: 10.1074/jbc.M110.216044
Uncoupling protein 3 (UCP3) modulates the activity of Sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) by decreasing mitochondrial ATP production
Abstract
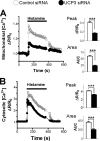
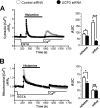
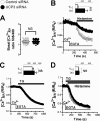
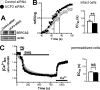
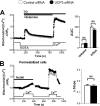
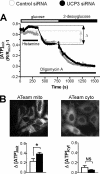
The uncoupling proteins UCP2 and UCP3 have been postulated to catalyze Ca(2+) entry across the inner membrane of mitochondria, but this proposal is disputed, and other, unrelated proteins have since been identified as the mitochondrial Ca(2+) uniporter. To clarify the role of UCPs in mitochondrial Ca(2+) handling, we down-regulated the expression of the only uncoupling protein of HeLa cells, UCP3, and measured Ca(2+) and ATP levels in the cytosol and in organelles with genetically encoded probes. UCP3 silencing did not alter mitochondrial Ca(2+) uptake in permeabilized cells. In intact cells, however, UCP3 depletion increased mitochondrial ATP production and strongly reduced the cytosolic and mitochondrial Ca(2+) elevations evoked by histamine. The reduced Ca(2+) elevations were due to inhibition of store-operated Ca(2+) entry and reduced depletion of endoplasmic reticulum (ER) Ca(2+) stores. UCP3 depletion accelerated the ER Ca(2+) refilling kinetics, indicating that the activity of sarco/endoplasmic reticulum Ca(2+) (SERCA) pumps was increased. Accordingly, SERCA inhibitors reversed the effects of UCP3 depletion on cytosolic, ER, and mitochondrial Ca(2+) responses. Our results indicate that UCP3 is not a mitochondrial Ca(2+) uniporter and that it instead negatively modulates the activity of SERCA by limiting mitochondrial ATP production. The effects of UCP3 on mitochondrial Ca(2+) thus reflect metabolic alterations that impact on cellular Ca(2+) homeostasis. The sensitivity of SERCA to mitochondrial ATP production suggests that mitochondria control the local ATP availability at ER Ca(2+) uptake and release sites.
Figures
Similar articles
-
Molecularly distinct routes of mitochondrial Ca2+ uptake are activated depending on the activity of the sarco/endoplasmic reticulum Ca2+ ATPase (SERCA).J Biol Chem. 2013 May 24;288(21):15367-79. doi: 10.1074/jbc.M113.462259. Epub 2013 Apr 16. J Biol Chem. 2013. PMID: 23592775 Free PMC article.
-
STIM1 knockdown reveals that store-operated Ca2+ channels located close to sarco/endoplasmic Ca2+ ATPases (SERCA) pumps silently refill the endoplasmic reticulum.J Biol Chem. 2007 Apr 13;282(15):11456-64. doi: 10.1074/jbc.M609551200. Epub 2007 Feb 5. J Biol Chem. 2007. PMID: 17283081
-
Glucose and endoplasmic reticulum calcium channels regulate HIF-1beta via presenilin in pancreatic beta-cells.J Biol Chem. 2008 Apr 11;283(15):9909-16. doi: 10.1074/jbc.M710601200. Epub 2008 Jan 3. J Biol Chem. 2008. PMID: 18174159
-
Sarco-Endoplasmic Reticulum Calcium Release Model Based on Changes in the Luminal Calcium Content.Adv Exp Med Biol. 2020;1131:337-370. doi: 10.1007/978-3-030-12457-1_14. Adv Exp Med Biol. 2020. PMID: 31646517 Review.
-
Privileged coupling between Ca(2+) entry through plasma membrane store-operated Ca(2+) channels and the endoplasmic reticulum Ca(2+) pump.Mol Cell Endocrinol. 2012 Apr 28;353(1-2):37-44. doi: 10.1016/j.mce.2011.08.021. Epub 2011 Aug 24. Mol Cell Endocrinol. 2012. PMID: 21878366 Review.
Cited by
-
The antidepressant fluoxetine induces necrosis by energy depletion and mitochondrial calcium overload.Oncotarget. 2017 Jan 10;8(2):3181-3196. doi: 10.18632/oncotarget.13689. Oncotarget. 2017. PMID: 27911858 Free PMC article.
-
UCP3 Regulates Single-Channel Activity of the Cardiac mCa1.J Membr Biol. 2016 Aug;249(4):577-84. doi: 10.1007/s00232-016-9913-2. Epub 2016 Jul 1. J Membr Biol. 2016. PMID: 27371160 Free PMC article.
-
The Uncoupling Proteins: A Systematic Review on the Mechanism Used in the Prevention of Oxidative Stress.Antioxidants (Basel). 2022 Feb 6;11(2):322. doi: 10.3390/antiox11020322. Antioxidants (Basel). 2022. PMID: 35204205 Free PMC article. Review.
-
Sodium-glucose co-transporter 2 inhibition as a mitochondrial therapy for atrial fibrillation in patients with diabetes?Cardiovasc Diabetol. 2020 Jan 7;19(1):5. doi: 10.1186/s12933-019-0984-0. Cardiovasc Diabetol. 2020. PMID: 31910841 Free PMC article.
-
Mitochondrial dysfunction is a key link involved in the pathogenesis of sick sinus syndrome: a review.Front Cardiovasc Med. 2024 Oct 29;11:1488207. doi: 10.3389/fcvm.2024.1488207. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 39534498 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

