Modulating microtubule stability enhances the cytotoxic response of cancer cells to Paclitaxel
- PMID: 21775522
- PMCID: PMC3679477
- DOI: 10.1158/0008-5472.CAN-11-0025
Modulating microtubule stability enhances the cytotoxic response of cancer cells to Paclitaxel
Abstract
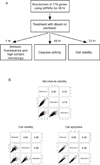
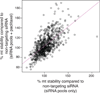
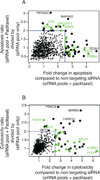
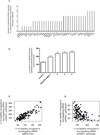
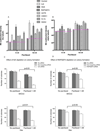
The extracellular matrix protein TGFBI enhances the cytotoxic response of cancer cells to paclitaxel by affecting integrin signals that stabilize microtubules. Extending the implications of this knowledge, we tested the more general hypothesis that cancer cell signals which increase microtubule stability before exposure to paclitaxel may increase its ability to stabilize microtubules and thereby enhance its cytotoxicity. Toward this end, we carried out an siRNA screen to evaluate how genetic depletion affected microtubule stabilization, cell viability, and apoptosis. High content microscopic analysis was carried out in the absence or presence of paclitaxel. Kinase knockdowns that stabilized microtubules strongly enhanced the effects of paclitaxel treatment. Conversely, kinase knockdowns that enhanced paclitaxel-mediated cytotoxicity sensitized cells to microtubule stabilization by paclitaxel. The siRNA screen identified several genes that have not been linked previously to microtubule regulation or paclitaxel response. Gene shaving and Bayesian resampling used to classify these genes suggested three pathways of paclitaxel-induced cell death related to apoptosis and microtubule stability, apoptosis alone, or neither process. Our results offer a functional classification of the genetic basis for paclitaxel sensitivity and they support the hypothesis that stabilizing microtubules prior to therapy could enhance antitumor responses to paclitaxel treatment.
©2011 AACR.
Conflict of interest statement
Figures

References
-
- Schiff PB, Horwitz SB. Taxol assembles tubulin in the absence of exogenous guanosine 5'-triphosphate or microtubule-associated proteins. Biochemistry. 1981;20:3247–3252. - PubMed
-
- Martin M, Rodriguez-Lescure A, Ruiz A, Alba E, Calvo L, Ruiz-Borrego M, et al. Randomized phase 3 trial of fluorouracil, epirubicin, and cyclophosphamide alone or followed by Paclitaxel for early breast cancer. J Natl Cancer Inst. 2008;100:805–814. - PubMed
-
- McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and cisplatin versus paclitaxel and cisplatin: a phase III randomized trial in patients with suboptimal stage III/IV ovarian cancer (from the Gynecologic Oncology Group) Semin Oncol. 1996;23:40–47. - PubMed
-
- Nogales E, Wolf SG, Khan IA, Luduena RF, Downing KH. Structure of tubulin at 6.5 A and location of the taxol-binding site. Nature. 1995;375:424–427. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

