Effect of sniffing on the temporal structure of mitral/tufted cell output from the olfactory bulb
- PMID: 21775605
- PMCID: PMC3159407
- DOI: 10.1523/JNEUROSCI.1805-11.2011
Effect of sniffing on the temporal structure of mitral/tufted cell output from the olfactory bulb
Abstract
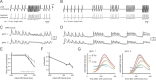
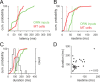
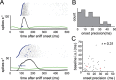
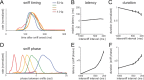
Neural activity underlying odor representations in the mammalian olfactory system is strongly patterned by respiratory behavior. These dynamics are central to many models of olfactory information processing. We have found previously that sensory inputs to the olfactory bulb change both their magnitude and temporal structure as a function of sniff frequency. Here, we ask how sniff frequency affects responses of mitral/tufted (MT) cells--the principal olfactory bulb output neurons. We recorded from MT cells in anesthetized rats while reproducing sniffs recorded previously from awake animals and varying sniff frequency. The dynamics of a sniff-evoked response were consistent from sniff to sniff but varied across cells. Compared to the dynamics of receptor neuron activation by the same sniffs, the MT response was shorter and faster, reflecting a temporal sharpening of sensory inputs. Increasing sniff frequency led to moderate attenuation of MT response magnitude and significant changes in the temporal structure of the sniff-evoked MT cell response. Most MT cells responded with a shorter duration and shorter rise-time spike burst as sniff frequency increased, reflecting increased temporal sharpening of inputs by the olfactory bulb. These temporal changes were necessary and sufficient to maintain respiratory modulation in the MT cell population across the range of sniff frequencies expressed during behavior. These results suggest that the input-output relationship in the olfactory bulb varies dynamically as a function of sniff frequency and that one function of the postsynaptic network is to maintain robust temporal encoding of odor information across different odor sampling strategies.
Figures



References
-
- Balu R, Larimer P, Strowbridge BW. Phasic stimuli evoke precisely timed spikes in intermittently discharging mitral cells. J Neurophysiol. 2004;92:743–753. - PubMed
-
- Bathellier B, Buhl DL, Accolla R, Carleton A. Dynamic ensemble odor coding in the mammalian olfactory bulb: sensory information at different timescales. Neuron. 2008;57:586–598. - PubMed
-
- Bhalla US, Bower JM. Multiday recordings from olfactory bulb neurons in awake freely moving rats: spatially and temporally organized variability in odorant response properties. J Comput Neurosci. 1997;4:221–256. - PubMed
-
- Chaput MA. Respiratory-phase-related coding of olfactory information in the olfactory bulb of awake freely-breathing rabbits. Physiol Behav. 1986;36:319–324. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
