Kinetics of hyperosmotically stimulated Na-K-2Cl cotransporter in Xenopus laevis oocytes
- PMID: 21775703
- PMCID: PMC4214853
- DOI: 10.1152/ajpcell.00131.2011
Kinetics of hyperosmotically stimulated Na-K-2Cl cotransporter in Xenopus laevis oocytes
Abstract
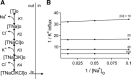
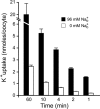
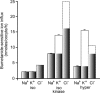
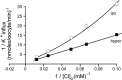
A detailed study of hypertonically stimulated Na-K-2Cl cotransport (NKCC1) in Xenopus laevis oocytes was carried out to better understand the 1 K(+):1 Cl(-) stoichiometry of transport that was previously observed. In this study, we derived the velocity equations for K(+) influx under both rapid equilibrium assumptions and combined equilibrium and steady-state assumptions and demonstrate that the behavior of the equations and curves in Lineweaver-Burke plots are consistent with a model where Cl(-) binds first, followed by Na(+), a second Cl(-), and then K(+). We further demonstrate that stimulation of K(+) movement by K(+) on the trans side is an intrinsic property of a carrier that transports multiple substrates. We also demonstrate that K(+) movement through NKCC1 is strictly dependent upon the presence of external Na(+), even though only a fraction of Na(+) is in fact transported. Finally, we propose that the larger transport of K(+), as compared with Na(+), is a result of the return of partially unloaded carriers, which masks the net 1Na(+):1K(+):2Cl(-) stoichiometry of NKCC1. These data have profound implications for the physiology of Na-K-2Cl cotransport, since transport of K-Cl in some conditions seems to be uncoupled from the transport of Na-Cl.
Figures






References
-
- Aiton JF, Brown CD, Ogden P, Simmons NL. K+ transport in “tight” epithelial monolayers of MDCK cells. J Membr Biol 65: 99–109, 1982 - PubMed
-
- Baudouin-Legros M, Brouillard F, Cougnon M, Tondelier D, Leclerc T, Edelman A. Modulation of CFTR gene expression in HT-29 cells by extracellular hyperosmolarity. Am J Physiol Cell Physiol 278: C49–C56, 2000 - PubMed
-
- Beck FX, Grünbein R, Lugmayr K, Neuhofer W. Heat shock proteins and the cellular response to osmotic stress. Cell Physiol Biochem 10: 303–306, 2000 - PubMed
-
- Benjamin BA, Johnson EA. A quantitative description of the Na-K-2Cl cotransporter and its conformity to experimental data. Am J Physiol Renal Physiol 273: F473–F482, 1997 - PubMed
-
- Brock TA, Brugnara C, Canessa M, Gimbrone MAJ. Bradykinin and vasopressin stimulate Na+-K+-Cl− cotransport in cultured endothelial cells. Am J Physiol Cell Physiol 250: C888–C895, 1986 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

