Prenatal nicotine increases matrix metalloproteinase 2 (MMP-2) expression in fetal guinea pig hearts
- PMID: 21775771
- PMCID: PMC3343142
- DOI: 10.1177/1933719111404605
Prenatal nicotine increases matrix metalloproteinase 2 (MMP-2) expression in fetal guinea pig hearts
Abstract
This study tested the hypothesis that maternal nicotine ingestion increases matrix metalloproteinase (MMP) expression in fetal hearts, which is mediated by the generation of reactive oxygen species. Timed pregnant guinea pigs were administered either water alone, nicotine (200 μg/mL), N-acetylcysteine (NAC), or nicotine plus NAC in their drinking water for 10 days at 52-day gestation (term = 65 days). Near-term (62 days), anesthetized fetuses were extracted, hearts were excised, and left cardiac ventricles snap frozen for analysis of MMP-2/-9/-13 protein and activity levels. Interstitial collagens were identified by Picrosirius red stain to assess changes in the extracellular matrix. Prenatal nicotine increased active MMP-2 forms and interstitial collagen but had no effect on either pro- or active MMP-9 or MMP-13 forms. In the presence of nicotine, NAC decreased active MMP-2 protein levels and reversed the nicotine-induced increase in collagen staining. We conclude that prenatal nicotine alters MMP-2 expression in fetal hearts that may be mediated by reactive oxygen species generation.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

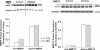


Similar articles
-
Maternal hypoxia alters matrix metalloproteinase expression patterns and causes cardiac remodeling in fetal and neonatal rats.Am J Physiol Heart Circ Physiol. 2011 Nov;301(5):H2113-21. doi: 10.1152/ajpheart.00356.2011. Epub 2011 Aug 19. Am J Physiol Heart Circ Physiol. 2011. PMID: 21856922 Free PMC article.
-
Chronic hypoxia increases peroxynitrite, MMP9 expression, and collagen accumulation in fetal guinea pig hearts.Pediatr Res. 2012 Jan;71(1):25-31. doi: 10.1038/pr.2011.10. Pediatr Res. 2012. PMID: 22289847
-
Differential expression of endothelial nitric oxide synthase in coronary and cardiac tissue in hypoxic fetal guinea pig hearts.J Soc Gynecol Investig. 2006 Oct;13(7):483-90. doi: 10.1016/j.jsgi.2006.06.005. Epub 2006 Sep 18. J Soc Gynecol Investig. 2006. PMID: 16979353
-
Intrauterine hypoxia upregulates proinflammatory cytokines and matrix metalloproteinases in fetal guinea pig hearts.Am J Obstet Gynecol. 2008 Jul;199(1):78.e1-6. doi: 10.1016/j.ajog.2007.12.004. Epub 2008 Feb 15. Am J Obstet Gynecol. 2008. PMID: 18279828
-
Chronic hypoxia decreases endothelial nitric oxide synthase protein expression in fetal guinea pig hearts.J Soc Gynecol Investig. 2005 Sep;12(6):388-95. doi: 10.1016/j.jsgi.2005.04.011. J Soc Gynecol Investig. 2005. PMID: 15982907
Cited by
-
Gestational Hypoxia and Developmental Plasticity.Physiol Rev. 2018 Jul 1;98(3):1241-1334. doi: 10.1152/physrev.00043.2017. Physiol Rev. 2018. PMID: 29717932 Free PMC article. Review.
-
Guinea pig models for translation of the developmental origins of health and disease hypothesis into the clinic.J Physiol. 2018 Dec;596(23):5535-5569. doi: 10.1113/JP274948. Epub 2018 May 30. J Physiol. 2018. PMID: 29633280 Free PMC article.
-
Prenatal Amino Acid Supplementation to Improve Fetal Growth: A Systematic Review and Meta-Analysis.Nutrients. 2020 Aug 21;12(9):2535. doi: 10.3390/nu12092535. Nutrients. 2020. PMID: 32825593 Free PMC article.
-
Differential responses of hippocampal neurons and astrocytes to nicotine and hypoxia in the fetal guinea pig.Neurotox Res. 2013 Jul;24(1):80-93. doi: 10.1007/s12640-012-9363-2. Epub 2012 Nov 29. Neurotox Res. 2013. PMID: 23192463 Free PMC article.
-
Differences of Matrix Metalloproteinase 2 Expression between Left and Right Ventricles in Response to Nandrolone Decanoate and/or Swimming Training in Mice.Chin Med J (Engl). 2018 Jan 20;131(2):207-212. doi: 10.4103/0366-6999.222330. Chin Med J (Engl). 2018. PMID: 29336370 Free PMC article.
References
-
- Substance Abuse and Mental Health Services Administration Results from the 2004 National Survey on Drug Use and Health: National Findings, Tobacco Use (PDF -1.17MB). Rockville, MD: Substance Abuse and Mental Health Services Administration, Office of Applied Studies; 2005
-
- Lambers DS, Clark KE. The maternal and fetal physiologic effects of nicotine. Semin Perinatol. 1996;20(2):115–126 - PubMed
-
- Slotkin TA. If nicotine is a developmental neurotoxicant in animal studies, dare we recommend nicotine replacement therapy in pregnant women and adolescents? Neurotoxicol Teratol. 2008;30(1):1–19 - PubMed
-
- Slotkin TA. Prenatal exposure to nicotine: what can we learn from animal models? In: Zagon IS, Slotkin TA, eds. Maternal Substance Abuse and The Developing Nervous System. San Diego, CA: Academic Press; 1992:97–124
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

