DMRT1 prevents female reprogramming in the postnatal mammalian testis
- PMID: 21775990
- PMCID: PMC3150961
- DOI: 10.1038/nature10239
DMRT1 prevents female reprogramming in the postnatal mammalian testis
Erratum in
- Nature. 2011 Sep 8;477(7363):238
Abstract
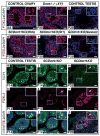
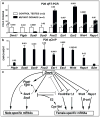
Sex in mammals is determined in the fetal gonad by the presence or absence of the Y chromosome gene Sry, which controls whether bipotential precursor cells differentiate into testicular Sertoli cells or ovarian granulosa cells. This pivotal decision in a single gonadal cell type ultimately controls sexual differentiation throughout the body. Sex determination can be viewed as a battle for primacy in the fetal gonad between a male regulatory gene network in which Sry activates Sox9 and a female network involving WNT/β-catenin signalling. In females the primary sex-determining decision is not final: loss of the FOXL2 transcription factor in adult granulosa cells can reprogram granulosa cells into Sertoli cells. Here we show that sexual fate is also surprisingly labile in the testis: loss of the DMRT1 transcription factor in mouse Sertoli cells, even in adults, activates Foxl2 and reprograms Sertoli cells into granulosa cells. In this environment, theca cells form, oestrogen is produced and germ cells appear feminized. Thus Dmrt1 is essential to maintain mammalian testis determination, and competing regulatory networks maintain gonadal sex long after the fetal choice between male and female. Dmrt1 and Foxl2 are conserved throughout vertebrates and Dmrt1-related sexual regulators are conserved throughout metazoans. Antagonism between Dmrt1 and Foxl2 for control of gonadal sex may therefore extend beyond mammals. Reprogramming due to loss of Dmrt1 also may help explain the aetiology of human syndromes linked to DMRT1, including disorders of sexual differentiation and testicular cancer.
Figures


Comment in
-
Re: DMRT1 prevents female reprogramming in the postnatal mammalian testis.J Urol. 2012 May;187(5):1924-5. doi: 10.1016/j.juro.2012.01.041. Epub 2012 Mar 17. J Urol. 2012. PMID: 22494762 No abstract available.
References
-
- Loffler KA, Zarkower D, Koopman P. Etiology of ovarian failure in blepharophimosis ptosis epicanthus inversus syndrome: FOXL2 is a conserved, early-acting gene in vertebrate ovarian development. Endocrinology. 2003;144:3237–3243. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

