The regulation of osteoclast function and bone resorption by small GTPases
- PMID: 21776413
- PMCID: PMC3136942
- DOI: 10.4161/sgtp.2.3.16453
The regulation of osteoclast function and bone resorption by small GTPases
Abstract
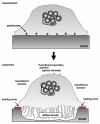
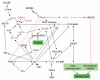
Osteoclasts are multinucleated cells that are responsible for resorption of bone, and increased activity of these cells is associated with several common bone diseases, including postmenopausal osteoporosis. Upon adhesion to bone, osteoclasts become polarized and reorganise their cytoskeleton and membrane to form unique domains including the sealing zone (SZ), which is a dense ring of F-actin-rich podosomes delimiting the ruffled border (RB), where protons and proteases are secreted to demineralise and degrade the bone matrix, respectively. These processes are dependent on the activity of small GTPases. Rho GTPases are well known to control the organization of F-actin and adhesion structures of different cell types, affecting subsequently their migration. In osteoclasts, RhoA, Rac, Cdc42, RhoU and also Arf6 regulate podosome assembly and their organization into the SZ. By contrast, the formation of the RB involves vesicular trafficking pathways that are regulated by the Rab family of GTPases, in particular lysosomal Rab7. Finally, osteoclast survival is dependent on the activity of Ras GTPases. The correct function of almost all these GTPases is absolutely dependent on post-translational prenylation, which enables them to localize to specific target membranes. Bisphosphonate drugs, which are widely used in the treatment of bone diseases such as osteoporosis, act by preventing the prenylation of small GTPases, resulting in the loss of the SZ and RB and therefore inhibition of osteoclast activity, as well as inducing osteoclast apoptosis. In this review we summarize current understanding of the role of specific prenylated small GTPases in osteoclast polarization, function and survival.
Figures


Similar articles
-
Modulation of osteoclast differentiation and bone resorption by Rho GTPases.Small GTPases. 2014;5:e28119. doi: 10.4161/sgtp.28119. Epub 2014 Mar 10. Small GTPases. 2014. PMID: 24614674 Free PMC article. Review.
-
The Roles of Small GTPases in Osteoclast Biology.Orthop Muscular Syst. 2014;3:1000161. doi: 10.4172/2161-0533.1000161. Orthop Muscular Syst. 2014. PMID: 25599004 Free PMC article.
-
Inhibition of protein prenylation by bisphosphonates causes sustained activation of Rac, Cdc42, and Rho GTPases.J Bone Miner Res. 2006 May;21(5):684-94. doi: 10.1359/jbmr.060118. J Bone Miner Res. 2006. PMID: 16734383
-
Podosome organization drives osteoclast-mediated bone resorption.Cell Adh Migr. 2014;8(3):191-204. doi: 10.4161/cam.27840. Cell Adh Migr. 2014. PMID: 24714644 Free PMC article. Review.
-
Impaired prenylation of Rab GTPases in the gunmetal mouse causes defects in bone cell function.Small GTPases. 2011 May;2(3):131-142. doi: 10.4161/sgtp.2.3.16488. Small GTPases. 2011. PMID: 21776414 Free PMC article.
Cited by
-
A Genome-Wide Association Study Identifies Multiple Regions Associated with Head Size in Catfish.G3 (Bethesda). 2016 Oct 13;6(10):3389-3398. doi: 10.1534/g3.116.032201. G3 (Bethesda). 2016. PMID: 27558670 Free PMC article.
-
The role of Rho GTPases' substrates Rac and Cdc42 in osteoclastogenesis and relevant natural medicinal products study.Biosci Rep. 2020 Jul 31;40(7):BSR20200407. doi: 10.1042/BSR20200407. Biosci Rep. 2020. PMID: 32578854 Free PMC article. Review.
-
Multiple Genetic Loci Associated with Pug Dog Thoracolumbar Myelopathy.Genes (Basel). 2023 Feb 1;14(2):385. doi: 10.3390/genes14020385. Genes (Basel). 2023. PMID: 36833311 Free PMC article.
-
Insights about the structure of farnesyl diphosphate synthase (FPPS) and the activity of bisphosphonates on the proliferation and ultrastructure of Leishmania and Giardia.Parasit Vectors. 2020 Apr 5;13(1):168. doi: 10.1186/s13071-020-04019-z. Parasit Vectors. 2020. PMID: 32248823 Free PMC article.
-
The relationship of blood CDC42 level with Th1 cells, Th17 cells, inflammation markers, disease risk/activity, and treatment efficacy of rheumatoid arthritis.Ir J Med Sci. 2022 Oct;191(5):2155-2161. doi: 10.1007/s11845-021-02858-y. Epub 2021 Dec 2. Ir J Med Sci. 2022. PMID: 34859333 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
