RASSF1A: Not a prototypical Ras effector
- PMID: 21776416
- PMCID: PMC3136945
- DOI: 10.4161/sgtp.2.3.16286
RASSF1A: Not a prototypical Ras effector
Abstract
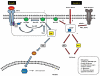
The Ras association domain family (RASSF) of genes are commonly silenced by promoter specific methylation in human cancers. After the cloning of the first two family members in early 2000 (RASSF1 and RASSF5), eight other related genes have been identified (RASSF2, 3, 4 and 6-10). The unifying motif amongst all RASSF family members is the presence of the Ras association (RA) domain that could potentially associate with the Ras family of GTPases. Detailed analyses have determined that RASSF family members are tumor suppressor proteins, activators of cell death, cell cycle modulators, microtubule stabilizers and possibly inflammatory mediators linked to NFκB. As such, exploring the biological function of this gene family is needed and if indeed RASSF proteins could be the missing link between Ras signaling and apoptosis. Several RASSF family members have been demonstrated to associate with Ras. However, there is still controversy regarding the ability of RASSF1A to utilize Ras to promote cell death and of the importance of the RASSF1A RA domain. The focus of this review is to highlight the importance of Ras binding to the RASSF family of proteins and discuss what we currently know about the biology of RASSF1A.
Figures


Similar articles
-
Colorectal cancer and RASSF family--a special emphasis on RASSF1A.Int J Cancer. 2013 Jan 15;132(2):251-8. doi: 10.1002/ijc.27696. Epub 2012 Jul 14. Int J Cancer. 2013. PMID: 22733432 Review.
-
The Ras-association domain family (RASSF) members and their role in human tumourigenesis.Biochim Biophys Acta. 2007 Sep;1776(1):58-85. doi: 10.1016/j.bbcan.2007.06.003. Epub 2007 Jul 4. Biochim Biophys Acta. 2007. PMID: 17692468 Free PMC article. Review.
-
Nore1 and RASSF1 regulation of cell proliferation and of the MST1/2 kinases.Methods Enzymol. 2006;407:290-310. doi: 10.1016/S0076-6879(05)07025-4. Methods Enzymol. 2006. PMID: 16757333
-
N-terminal RASSF family: RASSF7-RASSF10.Epigenetics. 2011 Mar;6(3):284-92. doi: 10.4161/epi.6.3.14108. Epub 2011 Mar 1. Epigenetics. 2011. PMID: 21116130 Free PMC article. Review.
-
The RASSF gene family members RASSF5, RASSF6 and RASSF7 show frequent DNA methylation in neuroblastoma.Mol Cancer. 2012 Jun 13;11:40. doi: 10.1186/1476-4598-11-40. Mol Cancer. 2012. PMID: 22695170 Free PMC article.
Cited by
-
Protein kinases of the Hippo pathway: regulation and substrates.Semin Cell Dev Biol. 2012 Sep;23(7):770-84. doi: 10.1016/j.semcdb.2012.07.002. Epub 2012 Aug 9. Semin Cell Dev Biol. 2012. PMID: 22898666 Free PMC article. Review.
-
Pathogenetic and Prognostic Significance of Inactivation of RASSF Proteins in Human Hepatocellular Carcinoma.Mol Biol Int. 2012;2012:849874. doi: 10.1155/2012/849874. Epub 2012 Apr 2. Mol Biol Int. 2012. PMID: 22548173 Free PMC article.
-
Differential methylation of the promoter and first exon of the RASSF1A gene in hepatocarcinogenesis.Hepatol Res. 2015 Nov;45(11):1110-23. doi: 10.1111/hepr.12449. Epub 2014 Dec 9. Hepatol Res. 2015. PMID: 25382672 Free PMC article.
-
Novel Biomarkers for Inflammatory Bowel Disease and Colorectal Cancer: An Interplay between Metabolic Dysregulation and Excessive Inflammation.Int J Mol Sci. 2023 Mar 22;24(6):5967. doi: 10.3390/ijms24065967. Int J Mol Sci. 2023. PMID: 36983040 Free PMC article.
-
Circulating Hypermethylated RASSF1A as a Molecular Biomarker for Diagnosis of Hepatocellular Carcinoma.Asian Pac J Cancer Prev. 2017 Jun 25;18(6):1637-1643. doi: 10.22034/APJCP.2017.18.6.1637. Asian Pac J Cancer Prev. 2017. PMID: 28670882 Free PMC article.
References
-
- Buday L, Downward J. Many faces of Ras activation. Biochim Biophys Acta. 2008;1786:178–187. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
