Plk2 Raps up Ras to subdue synapses
- PMID: 21776418
- PMCID: PMC3136947
- DOI: 10.4161/sgtp.2.3.16454
Plk2 Raps up Ras to subdue synapses
Abstract
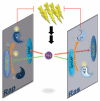
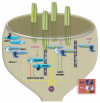
We recently identified the activity-inducible protein kinase Plk2 as a novel overseer of the balance between Ras and Rap small GTPases. Plk2 achieves a profound level of regulatory control by interacting with and phosphorylating at least four Ras and Rap guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs). Combined, these actions result in synergistic suppression of Ras and hyperstimulation of Rap signaling. Perturbation of Plk2 function abolished homeostatic adaptation of synapses to enhanced activity and impaired behavioral adaptation in various learning tasks, indicating that this regulation was critical for maintaining appropriate Ras/Rap levels. These studies provide insights into the highly cooperative nature of Ras and Rap regulation in neurons. However, different GEF and GAP substrates of Plk2 also controlled specific aspects of dendritic spine morphology, illustrating the ability of individual GAPs/GEFs to assemble microdomains of Ras and Rap signaling that respond to different stimuli and couple to distinct output pathways.
Figures
Similar articles
-
Requirement for Plk2 in orchestrated ras and rap signaling, homeostatic structural plasticity, and memory.Neuron. 2011 Mar 10;69(5):957-73. doi: 10.1016/j.neuron.2011.02.004. Neuron. 2011. PMID: 21382555 Free PMC article.
-
Phosphorylation of synaptic GTPase-activating protein (synGAP) by polo-like kinase (Plk2) alters the ratio of its GAP activity toward HRas, Rap1 and Rap2 GTPases.Biochem Biophys Res Commun. 2018 Sep 10;503(3):1599-1604. doi: 10.1016/j.bbrc.2018.07.087. Epub 2018 Jul 24. Biochem Biophys Res Commun. 2018. PMID: 30049443 Free PMC article.
-
Identification of guanine nucleotide exchange factors (GEFs) for the Rap1 GTPase. Regulation of MR-GEF by M-Ras-GTP interaction.J Biol Chem. 2000 Nov 10;275(45):34901-8. doi: 10.1074/jbc.M005327200. J Biol Chem. 2000. PMID: 10934204
-
Mechanisms for spatiotemporal regulation of Rho-GTPase signaling at synapses.Neurosci Lett. 2015 Aug 5;601:4-10. doi: 10.1016/j.neulet.2015.05.034. Epub 2015 May 21. Neurosci Lett. 2015. PMID: 26003445 Free PMC article. Review.
-
Regulation of Rap GTPases in mammalian neurons.Biol Chem. 2016 Oct 1;397(10):1055-69. doi: 10.1515/hsz-2016-0165. Biol Chem. 2016. PMID: 27186679 Review.
Cited by
-
Transcriptome profiling of the ventral pallidum reveals a role for pallido-thalamic neurons in cocaine reward.Mol Psychiatry. 2022 Oct;27(10):3980-3991. doi: 10.1038/s41380-022-01668-7. Epub 2022 Jun 28. Mol Psychiatry. 2022. PMID: 35764708 Free PMC article.
-
Polo-Like Kinase 2: From Principle to Practice.Front Oncol. 2022 Jul 8;12:956225. doi: 10.3389/fonc.2022.956225. eCollection 2022. Front Oncol. 2022. PMID: 35898867 Free PMC article. Review.
-
A tetra(ethylene glycol) derivative of benzothiazole aniline enhances Ras-mediated spinogenesis.J Neurosci. 2013 May 29;33(22):9306-18. doi: 10.1523/JNEUROSCI.1615-12.2013. J Neurosci. 2013. PMID: 23719799 Free PMC article.
-
Rap1 signaling prevents L-type calcium channel-dependent neurotransmitter release.J Neurosci. 2013 Apr 24;33(17):7245-52. doi: 10.1523/JNEUROSCI.5963-11.2013. J Neurosci. 2013. PMID: 23616533 Free PMC article.
-
Mammalian target of rapamycin complex 1 activation negatively regulates Polo-like kinase 2-mediated homeostatic compensation following neonatal seizures.Proc Natl Acad Sci U S A. 2013 Mar 26;110(13):5199-204. doi: 10.1073/pnas.1208010110. Epub 2013 Mar 11. Proc Natl Acad Sci U S A. 2013. PMID: 23479645 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
