Seeding specificity and ultrastructural characteristics of infectious recombinant prions
- PMID: 21776987
- PMCID: PMC3319715
- DOI: 10.1021/bi200786p
Seeding specificity and ultrastructural characteristics of infectious recombinant prions
Abstract
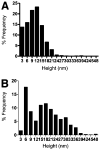
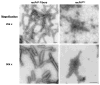
Infectious mouse prions can be produced from a mixture of bacterially expressed recombinant prion protein (recPrP), palmitoyloleoylphosphatidylglycerol (POPG), and RNA [Wang, F.; et al. (2010) Science 327, 1132]. In contrast, amyloid fibers produced from pure recPrP without POPG or RNA (recPrP fibers) fail to infect wild type mice [Colby, D.W.; et al. (2010) PLoS Pathog. 387, e1000736]. We compared the seeding specificity and ultrastructural features of infectious recombinant prions (recPrP(Sc)) with those of recPrP fibers. Our results indicate that PrP fibers are not able to induce the formation of PrP(Sc) molecules from wild type mouse brain homogenate substrate in serial protein misfolding cyclic amplification (sPMCA) reactions. Conversely, recPrP(Sc) molecules did not accelerate the formation of amyloid in vitro, under conditions that produce recPrP fibers spontaneously. Ultrastructurally, recombinant prions appear to be small spherical aggregates rather than elongated fibers, as determined by atomic force and electron microscopy. Taken together, our results show that recPrP(Sc) molecules and PrP fibers have different ultrastructural features and seeding specificities, suggesting that prion infectivity may be propagated by a specific and unique assembly pathway facilitated by cofactors.
© 2011 American Chemical Society
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

