The association of viral proteins with host cell dynein components during virus infection
- PMID: 21777384
- PMCID: PMC7164101
- DOI: 10.1111/j.1742-4658.2011.08252.x
The association of viral proteins with host cell dynein components during virus infection
Abstract
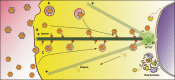
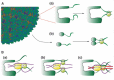
After fusion with the cellular plasma membrane or endosomal membranes, viral particles are generally too large to diffuse freely within the crowded cytoplasm environment. Thus, they will never reach the cell nucleus or the perinuclear areas where replication or reverse transcription usually takes place. It has been proposed that many unrelated viruses are transported along microtubules in a retrograde manner using the cellular dynein machinery or, at least, some dynein components. A putative employment of the dynein motor in a dynein-mediated transport has been suggested from experiments in which viral capsid proteins were used as bait in yeast two-hybrid screens using libraries composed of cellular proteins and dynein-associated chains were retrieved as virus-interacting proteins. In most cases DYNLL1, DYNLT1 or DYNLRB1 were identified as the dynein chains that interact with viral proteins. The importance of these dynein-virus interactions has been supported, in principle, by the observation that in some cases the dynein-interacting motifs of viral proteins altered by site-directed mutagenesis result in non-infective virions. Furthermore, overexpression of p50 dynamitin, which blocks the dynein-dynactin interaction, or incubation of infected cells with peptides that compete with viral polypeptides for dynein binding have been shown to alter the viral retrograde transport. Still, it remains to be proved that dynein light chains can bind simultaneously to incoming virions and to the dynein motor for retrograde transport to take place. In this review, we will analyse the association of viral proteins with dynein polypeptides and its implications for viral infection.
© 2011 The Authors Journal compilation © 2011 FEBS.
Figures
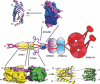
Similar articles
-
Eclipse phase of herpes simplex virus type 1 infection: Efficient dynein-mediated capsid transport without the small capsid protein VP26.J Virol. 2006 Aug;80(16):8211-24. doi: 10.1128/JVI.02528-05. J Virol. 2006. PMID: 16873277 Free PMC article.
-
Function of dynein and dynactin in herpes simplex virus capsid transport.Mol Biol Cell. 2002 Aug;13(8):2795-809. doi: 10.1091/mbc.01-07-0348. Mol Biol Cell. 2002. PMID: 12181347 Free PMC article.
-
Herpes simplex virus type 1 capsid protein VP26 interacts with dynein light chains RP3 and Tctex1 and plays a role in retrograde cellular transport.J Biol Chem. 2004 Jul 2;279(27):28522-30. doi: 10.1074/jbc.M311671200. Epub 2004 Apr 26. J Biol Chem. 2004. PMID: 15117959
-
Viral stop-and-go along microtubules: taking a ride with dynein and kinesins.Trends Microbiol. 2005 Jul;13(7):320-7. doi: 10.1016/j.tim.2005.05.010. Trends Microbiol. 2005. PMID: 15950476 Review.
-
Microtubule Retrograde Motors and Their Role in Retroviral Transport.Viruses. 2020 Apr 24;12(4):483. doi: 10.3390/v12040483. Viruses. 2020. PMID: 32344581 Free PMC article. Review.
Cited by
-
Molecular Basis for the Protein Recognition Specificity of the Dynein Light Chain DYNLT1/Tctex1: CHARACTERIZATION OF THE INTERACTION WITH ACTIVIN RECEPTOR IIB.J Biol Chem. 2016 Sep 30;291(40):20962-20975. doi: 10.1074/jbc.M116.736884. Epub 2016 Aug 8. J Biol Chem. 2016. PMID: 27502274 Free PMC article.
-
Dynein Regulators Are Important for Ecotropic Murine Leukemia Virus Infection.J Virol. 2016 Jul 11;90(15):6896-6905. doi: 10.1128/JVI.00863-16. Print 2016 Aug 1. J Virol. 2016. PMID: 27194765 Free PMC article.
-
HrpA anchors meningococci to the dynein motor and affects the balance between apoptosis and pyroptosis.J Biomed Sci. 2022 Jun 28;29(1):45. doi: 10.1186/s12929-022-00829-8. J Biomed Sci. 2022. PMID: 35765029 Free PMC article.
-
Proteomic snapshot of saliva samples predicts new pathways implicated in SARS-CoV-2 pathogenesis.Clin Proteomics. 2024 May 22;21(1):37. doi: 10.1186/s12014-024-09482-9. Clin Proteomics. 2024. PMID: 38778280 Free PMC article.
-
The Role of Host Cytoskeleton in Flavivirus Infection.Virol Sin. 2019 Feb;34(1):30-41. doi: 10.1007/s12250-019-00086-4. Epub 2019 Feb 6. Virol Sin. 2019. PMID: 30725318 Free PMC article. Review.
References
-
- Schliwa M & Woehlke G (2003) Molecular motors. Nature 422, 759–765. - PubMed
-
- Vale RD (2003) The molecular motor toolbox for intracellular transport. Cell 112, 467–480. - PubMed
-
- Sakakibara H & Oiwa K (2011) Molecular organization and force‐generating mechanism of dynein. FEBS J 278, 2964–2979. - PubMed
-
- Oiwa K & Sakakibara H (2005) Recent progress in dynein structure and mechanism. Curr Opin Cell Biol 17, 98–103. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

