Upregulation of cathepsin D in the caudate nucleus of primates with experimental parkinsonism
- PMID: 21777416
- PMCID: PMC3160400
- DOI: 10.1186/1750-1326-6-52
Upregulation of cathepsin D in the caudate nucleus of primates with experimental parkinsonism
Abstract
Background: In Parkinson's disease there is progressive loss of dopamine containing neurons in the substantia nigra pars compacta. The neuronal damage is not limited to the substantia nigra but progresses to other regions of brain, leading to loss of motor control as well as cognitive abnormalities. The purpose of this study was to examine causes of progressive damage in the caudate nucleus, which plays a major role in motor coordination and cognition, in experimental Parkinson's disease.
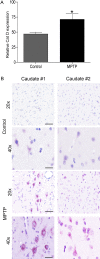
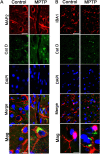
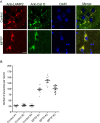
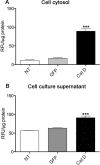
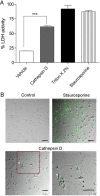
Results: Using chronic 1-methyl-4phenyl-1,2,3,6-tetrahydropyridine treatment of rhesus monkeys to model Parkinson's disease, we found a upregulation of Cathepsin D, a lysosomal aspartic protease, in the caudate nucleus of treated monkeys. Immunofluorescence analysis of caudate nucleus brain tissue showed that the number of lysosomes increased concurrently with the increase in Cathepsin D in neurons. In vitro overexpression of Cathepsin D in a human neuroblastoma cell line led to a significant increase in the number of the lysosomes. Such expression also resulted in extralysosomal Cathepsin D and was accompanied by significant neuronal death associated with caspase activation. We examined apoptotic markers and found a strong correlation of Cathepsin D overexpression to apoptosis.
Conclusions: Following damage to the substantia nigra resulting in experimental Parkinson's disease, we have identified pathological changes in the caudate nucleus, a likely site of changes leading to the progression of disease. Cathepsin D, implicated in pathogenic mechanisms in other disorders, was increased, and our in vitro studies revealed its overexpression leads to cellular damage and death. This work provides important clues to the progression of Parkinson's, and provides a new target for strategies to ameliorate the progression of this disease.
Figures

Similar articles
-
Early signs of neuronal apoptosis in the substantia nigra pars compacta of the progressive neurodegenerative mouse 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/probenecid model of Parkinson's disease.Neuroscience. 2006 Jun 19;140(1):67-76. doi: 10.1016/j.neuroscience.2006.02.007. Epub 2006 Mar 14. Neuroscience. 2006. PMID: 16533572
-
Free-water imaging in Parkinson's disease and atypical parkinsonism.Brain. 2016 Feb;139(Pt 2):495-508. doi: 10.1093/brain/awv361. Epub 2015 Dec 24. Brain. 2016. PMID: 26705348 Free PMC article.
-
Upregulation of Cysteine Protease Cathepsin X in the 6-Hydroxydopamine Model of Parkinson's Disease.Front Mol Neurosci. 2018 Nov 2;11:412. doi: 10.3389/fnmol.2018.00412. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30450037 Free PMC article.
-
Post mortem studies in Parkinson's disease--is it possible to detect brain areas for specific symptoms?J Neural Transm Suppl. 1999;56:1-29. doi: 10.1007/978-3-7091-6360-3_1. J Neural Transm Suppl. 1999. PMID: 10370901 Review.
-
Age and Parkinson's disease-related neuronal death in the substantia nigra pars compacta.J Neural Transm Suppl. 2009;(73):203-13. doi: 10.1007/978-3-211-92660-4_16. J Neural Transm Suppl. 2009. PMID: 20411779 Review.
Cited by
-
Echinacoside protects against MPTP/MPP+-induced neurotoxicity via regulating autophagy pathway mediated by Sirt1.Metab Brain Dis. 2019 Feb;34(1):203-212. doi: 10.1007/s11011-018-0330-3. Epub 2018 Nov 13. Metab Brain Dis. 2019. PMID: 30426321 Free PMC article.
-
The Effect of Fucoidan on Cellular Oxidative Stress and the CatD-Bax Signaling Axis in MN9D Cells Damaged by 1-Methyl-4-Phenypyridinium.Front Aging Neurosci. 2019 Jan 16;10:429. doi: 10.3389/fnagi.2018.00429. eCollection 2018. Front Aging Neurosci. 2019. PMID: 30700973 Free PMC article.
-
Resveratrol induces autophagic apoptosis via the lysosomal cathepsin D pathway in human drug-resistant K562/ADM leukemia cells.Exp Ther Med. 2018 Mar;15(3):3012-3019. doi: 10.3892/etm.2018.5742. Epub 2018 Jan 12. Exp Ther Med. 2018. PMID: 29456707 Free PMC article.
-
Mannose 6-Phosphate Receptor Is Reduced in -Synuclein Overexpressing Models of Parkinsons Disease.PLoS One. 2016 Aug 10;11(8):e0160501. doi: 10.1371/journal.pone.0160501. eCollection 2016. PLoS One. 2016. PMID: 27509067 Free PMC article.
-
New roles of glycosaminoglycans in α-synuclein aggregation in a cellular model of Parkinson disease.PLoS One. 2015 Jan 24;10(1):e0116641. doi: 10.1371/journal.pone.0116641. eCollection 2015. PLoS One. 2015. PMID: 25617759 Free PMC article.
References
-
- Marklund P, Larsson A, Elgh E, Linder J, Riklund KA, Forsgren L, Nyberg L. Temporal dynamics of basal ganglia under-recruitment in Parkinson's disease: transient caudate abnormalities during updating of working memory. Brain. 2009;132:336–346. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

