Inverse zonation of hepatocyte transduction with AAV vectors between mice and non-human primates
- PMID: 21778099
- PMCID: PMC3269907
- DOI: 10.1016/j.ymgme.2011.06.002
Inverse zonation of hepatocyte transduction with AAV vectors between mice and non-human primates
Abstract
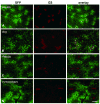
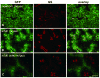
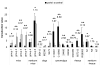
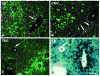
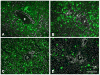
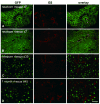
Gene transfer vectors based on adeno-associated virus 8 (AAV8) are highly efficient in liver transduction and can be easily administered by intravenous injection. In mice, AAV8 transduces predominantly hepatocytes near central veins and yields lower transduction levels in hepatocytes in periportal regions. This transduction bias has important implications for gene therapy that aims to correct metabolic liver enzymes because metabolic zonation along the porto-central axis requires the expression of therapeutic proteins within the zone where they are normally localized. In the present study we compared the expression pattern of AAV8 expressing green fluorescent protein (GFP) in liver between mice, dogs, and non-human primates. We confirmed the pericentral dominance in transgene expression in mice with AAV8 when the liver-specific thyroid hormone-binding globulin (TBG) promoter was used but also observed the same expression pattern with the ubiquitous chicken β-actin (CB) and cytomegalovirus (CMV) promoters, suggesting that transduction zonation is not caused by promoter specificity. Predominantly pericentral expression was also found in dogs injected with AAV8. In contrast, in cynomolgus and rhesus macaques the expression pattern from AAV vectors was reversed, i.e. transgene expression was most intense around portal areas and less intense or absent around central veins. Infant rhesus macaques as well as newborn mice injected with AAV8 however showed a random distribution of transgene expression with neither portal nor central transduction bias. Based on the data in monkeys, adult humans treated with AAV vectors are predicted to also express transgenes predominantly in periportal regions whereas infants are likely to show a uniform transduction pattern in liver.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
References
-
- Gao G, Lu Y, Calcedo R, Grant RL, Bell P, Wang L, Figueredo J, Lock M, Wilson JM. Biology of AAV serotype vectors in liver-directed gene transfer to nonhuman primates. Mol Ther. 2006;13:77–87. - PubMed
-
- Katz NR. Metabolic heterogeneity of hepatocytes across the liver acinus. J Nutr. 1992;122:843–849. - PubMed
-
- Gebhardt R. Metabolic zonation of the liver: regulation and implications for liver function. Pharmacol Ther. 1992;53:275–354. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1RR031988/RR/NCRR NIH HHS/United States
- RR00169/RR/NCRR NIH HHS/United States
- RR02512/RR/NCRR NIH HHS/United States
- P40 RR002512/RR/NCRR NIH HHS/United States
- HD057247/HD/NICHD NIH HHS/United States
- P01 HD057247/HD/NICHD NIH HHS/United States
- P51 RR000169/RR/NCRR NIH HHS/United States
- DK047757/DK/NIDDK NIH HHS/United States
- UL1 RR031988/RR/NCRR NIH HHS/United States
- R24 HL085794/HL/NHLBI NIH HHS/United States
- P30HD40677/HD/NICHD NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
- P30 HD040677/HD/NICHD NIH HHS/United States
- R01 DK054481/DK/NIDDK NIH HHS/United States
- P30 DK047757/DK/NIDDK NIH HHS/United States
- DK54481/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

