Cloning and molecular characterization of a mitogen-activated protein kinase gene from Poncirus trifoliata whose ectopic expression confers dehydration/drought tolerance in transgenic tobacco
- PMID: 21778184
- PMCID: PMC3193021
- DOI: 10.1093/jxb/err229
Cloning and molecular characterization of a mitogen-activated protein kinase gene from Poncirus trifoliata whose ectopic expression confers dehydration/drought tolerance in transgenic tobacco
Abstract
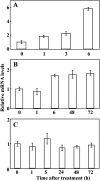
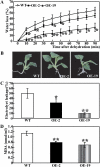
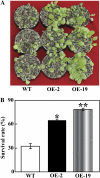
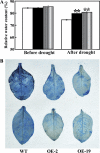
The mitogen-activated protein kinase (MAPK) cascade plays pivotal roles in diverse signalling pathways related to plant development and stress responses. In this study, the cloning and functional characterization of a group-I MAPK gene, PtrMAPK, in Poncirus trifoliata (L.) Raf are reported. PtrMAPK contains 11 highly conserved kinase domains and a phosphorylation motif (TEY), and is localized in the nucleus of transformed onion epidermal cells. The PtrMAPK transcript level was increased by dehydration and cold, but was unaffected by salt. Transgenic overexpression of PtrMAPK in tobacco confers dehydration and drought tolerance. The transgenic plants exhibited better water status, less reactive oxygen species (ROS) generation, and higher levels of antioxidant enzyme activity and metabolites than the wild type. Interestingly, the stress tolerance capacity of the transgenic plants was compromised by inhibitors of antioxidant enzymes. In addition, overexpression of PtrMAPK enhanced the expression of ROS-related and stress-responsive genes under normal or drought conditions. Taken together, these data demonstrate that PtrMAPK acts as a positive regulator in dehydration/drought stress responses by either regulating ROS homeostasis through activation of the cellular antioxidant systems or modulating transcriptional levels of a variety of stress-associated genes.
Figures









References
-
- Aswath CR, Kim SH, Mo SY, Kim DH. Transgenic plants of creeping bent grass harboring the stress inducible gene, 9-cis-epoxycarotenoid dioxygenase, are highly tolerant to drought and NaCl stress. Plant Growth Regulation. 2005;47:129–139.
-
- Buchanan BB, Balmer Y. Redox regulation: a broadening horizon. Annual Review of Plant Biology. 2005;56:187–220. - PubMed
-
- Eichberg J, Iyer S. Phosphorylation of myelin protein: recent advances. Neurochemical Research. 1996;21:527–535. - PubMed
-
- Feilner T, Hultschig C, Lee J, et al. High throughput identification of potential Arabidopsis mitogen-activated protein kinases substrates. Molecular and Cellular Proteomics. 2005;4:1558–1568. - PubMed
-
- Fiil BK, Petersen K, Petersen M, Mundy J. Gene regulation by MAP kinase cascades. Current Opinion in Plant Biology. 2009;12:615–621. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

