Alternatively spliced androgen receptor variants
- PMID: 21778211
- PMCID: PMC3235645
- DOI: 10.1530/ERC-11-0141
Alternatively spliced androgen receptor variants
Abstract
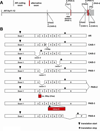
Alternative splicing is an important mechanism for increasing functional diversity from a limited set of genes. Deregulation of this process is common in diverse pathologic conditions. The androgen receptor (AR) is a steroid receptor transcription factor with functions critical for normal male development as well as the growth and survival of normal and cancerous prostate tissue. Studies of AR function in androgen insensitivity syndrome (AIS) and prostate cancer (PCa) have demonstrated loss-of-function AR alterations in AIS and gain-of-function AR alterations in PCa. Over the past two decades, AR gene alterations have been identified in various individuals with AIS, which disrupt normal AR splicing patterns and yield dysfunctional AR protein variants. Recently, altered AR splicing patterns have been identified as a mechanism of PCa progression and resistance to androgen depletion therapy. Several studies have described the synthesis of alternatively spliced transcripts encoding truncated AR isoforms that lack the ligand-binding domain, which is the ultimate target of androgen depletion. Many of these truncated AR isoforms function as constitutively active, ligand-independent transcription factors that can support androgen-independent expression of AR target genes, as well as the androgen-independent growth of PCa cells. In this review, we will summarize the various alternatively spliced AR variants that have been discovered, with a focus on their role and origin in the pathologic conditions of AIS and PCa.
Figures



References
-
- Agoulnik IU, Weigel NL. Androgen receptor action in hormone-dependent and recurrent prostate cancer. J Cell Biochem. 2006;99:362–372. - PubMed
-
- Ahrens-Fath I, Politz O, Geserick C, Haendler B. Androgen receptor function is modulated by the tissue-specific AR45 variant. FEBS J. 2005;272:74–84. - PubMed
-
- Attard G, Reid AH, Olmos D, de Bono JS. Antitumor activity with CYP17 blockade indicates that castration-resistant prostate cancer frequently remains hormone driven. Cancer Res. 2009;69:4937–4940. - PubMed
-
- Bain DL, Heneghan AF, Connaghan-Jones KD, Miura MT. Nuclear Receptor Structure: Implications for Function. Annu Rev Physiol. 2006;69 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

