Cigarette smoke induces DNA damage and alters base-excision repair and tau levels in the brain of neonatal mice
- PMID: 21778470
- PMCID: PMC3179679
- DOI: 10.1093/toxsci/kfr187
Cigarette smoke induces DNA damage and alters base-excision repair and tau levels in the brain of neonatal mice
Abstract
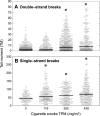
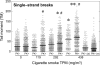
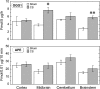
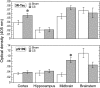
The prenatal and perinatal periods of brain development are especially vulnerable to insults by environmental agents. Early life exposure to cigarette smoke (CS), which contains both genotoxicants and oxidants, is considered an important risk factor for both neurodevelopmental and neurodegenerative disorders. Yet, little is known regarding the underlying pathogenetic mechanisms. In the present study, neonatal Swiss ICR (CD-1) albino mice were exposed to various concentrations of CS for 4 weeks and the brain examined for lipid peroxides, DNA damage, base-excision repair (BER) enzymes, apoptosis, and levels of the microtubule protein tau. CS induced a dose-dependent increase in both malondialdehyde and various types of DNA damage, including single-strand breaks, double-strand breaks, and DNA-protein cross-links. However, the CS-induced DNA damage in the brain returned to basal levels 1 week after smoking cessation. CS also modulated the activity and distribution of the BER enzymes 8-oxoguanine-DNA-glycosylase (OGG1) and apyrimidinic/apurinic endonuclease (APE1) in several brain regions. Normal tau (i.e., three-repeat tau, 3R tau) and various pathological forms of tau were also measured in the brain of CS-exposed neonatal mice, but only 3R tau and tau phosphorylated at serine 199 were significantly elevated. The oxidative stress, genomic dysregulation, and alterations in tau metabolism caused by CS during a critical period of brain development could explain why CS is an important risk factor for both neurodevelopmental and neurodegenerative disorders appearing in later life.
Figures

Similar articles
-
Oxidative stress alters base excision repair pathway and increases apoptotic response in apurinic/apyrimidinic endonuclease 1/redox factor-1 haploinsufficient mice.Free Radic Biol Med. 2009 Jun 1;46(11):1488-99. doi: 10.1016/j.freeradbiomed.2009.02.021. Epub 2009 Mar 3. Free Radic Biol Med. 2009. PMID: 19268524 Free PMC article.
-
The role of the N-terminal domain of human apurinic/apyrimidinic endonuclease 1, APE1, in DNA glycosylase stimulation.DNA Repair (Amst). 2018 Apr;64:10-25. doi: 10.1016/j.dnarep.2018.02.001. Epub 2018 Feb 11. DNA Repair (Amst). 2018. PMID: 29475157
-
Oxidative DNA damage is concurrently repaired by base excision repair (BER) and apyrimidinic endonuclease 1 (APE1)-initiated nonhomologous end joining (NHEJ) in cortical neurons.Neuropathol Appl Neurobiol. 2020 Jun;46(4):375-390. doi: 10.1111/nan.12584. Epub 2019 Nov 6. Neuropathol Appl Neurobiol. 2020. PMID: 31628877 Free PMC article.
-
Fine-tuning of DNA base excision/strand break repair via acetylation.DNA Repair (Amst). 2020 Sep;93:102931. doi: 10.1016/j.dnarep.2020.102931. DNA Repair (Amst). 2020. PMID: 33087268 Free PMC article. Review.
-
Recent advances in biosensor for DNA glycosylase activity detection.Talanta. 2022 Mar 1;239:123144. doi: 10.1016/j.talanta.2021.123144. Epub 2021 Dec 15. Talanta. 2022. PMID: 34923254 Review.
Cited by
-
Cigarette smoke sustains immunosuppressive microenvironment inducing M2 macrophage polarization and viability in lung cancer settings.PLoS One. 2024 May 22;19(5):e0303875. doi: 10.1371/journal.pone.0303875. eCollection 2024. PLoS One. 2024. PMID: 38776331 Free PMC article.
-
Smoking and Neuropsychiatric Disease-Associations and Underlying Mechanisms.Int J Mol Sci. 2021 Jul 6;22(14):7272. doi: 10.3390/ijms22147272. Int J Mol Sci. 2021. PMID: 34298890 Free PMC article. Review.
-
Contribution of HOGG1 Ser³²⁶Cys polymorphism to the development of prostate cancer in smokers: meta-analysis of 2779 cases and 3484 controls.PLoS One. 2012;7(1):e30309. doi: 10.1371/journal.pone.0030309. Epub 2012 Jan 18. PLoS One. 2012. PMID: 22279581 Free PMC article.
-
Association of XRCC3 and XRCC4 gene polymorphisms, family history of cancer and tobacco smoking with non-small-cell lung cancer in a Chinese population: a case-control study.J Hum Genet. 2013 Oct;58(10):679-85. doi: 10.1038/jhg.2013.78. Epub 2013 Aug 8. J Hum Genet. 2013. PMID: 23924833 Free PMC article.
-
[Correlation between drinking behavior and polymorphisms of extracellular superoxide dismutase, aldehyde dehydrogenase 2 genes, and oral squamous cell carcinoma].Hua Xi Kou Qiang Yi Xue Za Zhi. 2014 Apr;32(2):119-24. doi: 10.7518/hxkq.2014.02.004. Hua Xi Kou Qiang Yi Xue Za Zhi. 2014. PMID: 24881203 Free PMC article. Chinese.
References
-
- Almeida OP, Garrido GJ, Lautenschlager NT, Hulse GK, Jamrozik K, Flicker L. Smoking is associated with reduced cortical regional gray matter density in brain regions associated with incipient Alzheimer disease. Am. J. Geriatr. Psychiatry. 2008;16:92–98. - PubMed
-
- Balansky R, Ganchev G, Iltcheva M, Steele VE, D'Agostini F, De Flora S. Potent carcinogenicity of cigarette smoke in mice exposed early in life. Carcinogenesis. 2007;28:2236–2243. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

