Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson's disease
- PMID: 21778691
- PMCID: PMC3699815
- DOI: 10.1159/000328516
Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson's disease
Abstract
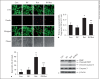
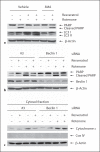
Excessive misfolded proteins and/or dysfunctional mitochondria, which may cause energy deficiency, have been implicated in the etiopathogenesis of Parkinson's disease (PD). Enhanced clearance of misfolded proteins or injured mitochondria via autophagy has been reported to have neuroprotective roles in PD models. The fact that resveratrol is a known compound with multiple beneficial effects similar to those associated with energy metabolism led us to explore whether neuroprotective effects of resveratrol are related to its role in autophagy regulation. We tested whether modulation of mammalian silent information regulator 2 (SIRT1) and/or metabolic energy sensor AMP-activated protein kinase (AMPK) are involved in autophagy induction by resveratrol, leading to neuronal survival. Our results showed that resveratrol protected against rotenone-induced apoptosis in SH-SY5Y cells and enhanced degradation of α-synucleins in α-synuclein-expressing PC12 cell lines via autophagy induction. We found that suppression of AMPK and/or SIRT1 caused decrease of protein level of LC3-II, indicating that AMPK and/or SIRT1 are required in resveratrol-mediated autophagy induction. Moreover, suppression of AMPK caused inhibition of SIRT1 activity and attenuated protective effects of resveratrol on rotenone-induced apoptosis, further suggesting that AMPK-SIRT1-autophagy pathway plays an important role in the neuroprotection by resveratrol on PD cellular models.
Copyright © 2011 S. Karger AG, Basel.
Figures





References
-
- Kopin IJ, Markey SP. MPTP toxicity, implications for research in Parkinson's disease. Annu Rev Neurosci. 1988;11:81–96. - PubMed
-
- Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. - PubMed
-
- Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, González-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, Wood NW. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science. 2004;304:1158–1160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

