Rotavirus stimulates release of serotonin (5-HT) from human enterochromaffin cells and activates brain structures involved in nausea and vomiting
- PMID: 21779163
- PMCID: PMC3136449
- DOI: 10.1371/journal.ppat.1002115
Rotavirus stimulates release of serotonin (5-HT) from human enterochromaffin cells and activates brain structures involved in nausea and vomiting
Abstract
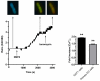
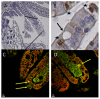
Rotavirus (RV) is the major cause of severe gastroenteritis in young children. A virus-encoded enterotoxin, NSP4 is proposed to play a major role in causing RV diarrhoea but how RV can induce emesis, a hallmark of the illness, remains unresolved. In this study we have addressed the hypothesis that RV-induced secretion of serotonin (5-hydroxytryptamine, 5-HT) by enterochromaffin (EC) cells plays a key role in the emetic reflex during RV infection resulting in activation of vagal afferent nerves connected to nucleus of the solitary tract (NTS) and area postrema in the brain stem, structures associated with nausea and vomiting. Our experiments revealed that RV can infect and replicate in human EC tumor cells ex vivo and in vitro and are localized to both EC cells and infected enterocytes in the close vicinity of EC cells in the jejunum of infected mice. Purified NSP4, but not purified virus particles, evoked release of 5-HT within 60 minutes and increased the intracellular Ca²⁺ concentration in a human midgut carcinoid EC cell line (GOT1) and ex vivo in human primary carcinoid EC cells concomitant with the release of 5-HT. Furthermore, NSP4 stimulated a modest production of inositol 1,4,5-triphosphate (IP₃), but not of cAMP. RV infection in mice induced Fos expression in the NTS, as seen in animals which vomit after administration of chemotherapeutic drugs. The demonstration that RV can stimulate EC cells leads us to propose that RV disease includes participation of 5-HT, EC cells, the enteric nervous system and activation of vagal afferent nerves to brain structures associated with nausea and vomiting. This hypothesis is supported by treating vomiting in children with acute gastroenteritis with 5-HT₃ receptor antagonists.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

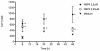
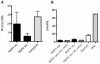


References
-
- Widdowson MA, Steele D, Vojdani J, Wecker J, Parashar U. Global rotavirus surveillance: determining the need and measuring the impact of rotavirus vaccines. J Infect Dis. 2009;200(Suppl 1):S1–8. - PubMed
-
- Lundgren O, Peregrin AT, Persson K, Kordasti S, Uhnoo I, et al. Role of the enteric nervous system in the fluid and electrolyte secretion of rotavirus diarrhea. Science. 2000;287:491–495. - PubMed
-
- Ball JM, Tian P, Zeng CQ, Morris AP, Estes MK. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science. 1996;272:101–104. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

