Development of the S-303 Pathogen Inactivation Technology for Red Blood Cell Concentrates
- PMID: 21779204
- PMCID: PMC3132978
- DOI: 10.1159/000324458
Development of the S-303 Pathogen Inactivation Technology for Red Blood Cell Concentrates
Abstract
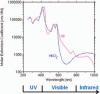
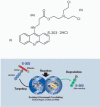
Pathogen inactivation systems are in use in many European countries as routine procedures. However, a pathogen inactivation system for erythrocytes is currently not available. Although significant improvements have been made to decrease the incidence of transfusion-transmitted infections, risks remain for infectious disease agents specific to red blood cell concentrates, such as parasitic infections resulting in babesiosis and malaria. The pathogen inactivation system for erythrocytes utilizes S-303 and glutathione for the treatment of red blood cell concentrates. Preclinical studies to assess the pathogen inactivation efficacy and toxicology as well as preliminary clinical studies have been completed. Preclinical studies have shown log reduction for leukocytes, several viruses and bacteria in excess of 4 to 6 logs. Preclinical toxicology studies were conducted to enable the initiation of two phase III clinical studies in the USA for support of acute and chronic anemia. A second-generation system was developed after observation of an unexpected immune response in two chronic anemia patients. Preclinical pathogen inactivation studies, serological evaluations and a clinical study to evaluate survival of S-303-treated erythrocytes have been completed to support advanced development of the S-303 pathogen inactivation system. A functional system for the inactivation of red blood cell concentrates has been completed and is reaching clinical application.
Technologien zur Pathogeninaktivierung sind in zahlreichen europäischen Ländern als Routineverfahren etabliert, jedoch fehlt ein solches für Erythrozyten. Auch vor dem Hintergrund signifikanter Verbesserungen bei der Testung von Blutkomponenten verbleiben Restrisiken, einschließlich von Pathogenen, wie etwa im Bereich der auch durch Erythrozyten übertragenen Parasiten oder von Bakterien. Das Pathogeninaktivierungssystem für Erythrozyten nutzt S-303 und Glutathion im funktionell geschlossenen System. Präklinische Studien haben eine Reduktion von Leukozyten, mehreren Modellviren und von Bakterien um 4–6 Log-Stufen belegt. Präklinische toxikologische Studien wurden durchgeführt und haben zur Initiierung von Phase-III-Studien bei akuter und chronischer Anämie in den USA geführt. Nach Immunantworten in 2 Patienten gegen pathogeninaktivierte Erythrozyten wurde eine neue Generation des Inaktivie-rungssystems entwickelt. Präklinische Inaktivierungs-studien, serologische Evaluationen und eine klinische Studie zur Ermittlung der Überlebensrate von S-303-behandelten Erythrozyten liegen vor; die Daten unter-stützen die Weiterentwicklung des Pathogeninaktivie-rungssystems. Ein funktionelles System zur Inaktivie-rung von Erythrozytenkonzentraten hat die präklinische Entwicklung komplettiert und die erste klinische Anwendung erreicht.
Figures


References
-
- van Rhenen D, Gulliksson H, Cazenave JP, Pamphilon D, Ljungman P, Kluter H, Venneij H, Kappers-Klunne M, de Greef G, Laforet M, Lioure B, Davis K, Marblie S, Mayaudon V, Rament J, Conlan M, Lin L, Metzel P, Buchholz D, Corash L. Transfusion of pooled buffy coat platelet components prepared with photochemical pathogen in-activation treatment: the euroSPRITE trial. Blood. 2003;101:2426–2433. - PubMed
-
- McCullough J, Vesole DH, Benjamin RJ, Slichter SJ, Pineda A, Snyder E, Stadtmauer EA, Lopez-Plaza I, Coutre S, Strauss RG, Goodnough LT, Fridey JL, Raife T, Cable R, Murphy S, Howard F, Davis K, Lin JS, Metzel P, Lin L, Koutsoukos A, Corash L, Buchholz DH, Conlan MG. Therapeutic efficacy and safety of platelets treated with a photochemical process for pathogen inactivation: the SPRINT trial. Blood. 2004;104:1534–1541. - PubMed
-
- Janetzko K, Cazenave JP, Klüter H, Kientz D, Michel M, Beris P, Lioure B, Hastka J, Marblie S, Mayaudon V, Lin L, Lin JS, Conlan MG, Rament J. Therapeutic efficacy and safety of photochemically treated apheresis platelets processed with an optimized integrated set. Transfusion. 2005;45:1443–1452. - PubMed
-
- Klein HG, Anderson D, Bernardi MJ, Cable R, Carey W, Hoch JS, Robitaille N, Sivilotti ML, Smaill F. Pathogen inactivation: making decisions about new technologies. Report of a consensus conference. Transfusion. 2007;47:2338–2347. - PubMed
-
- Mintz PD, Bass NM, Petz LD, Steadman R, Streiff M, McCullough J, Burks S, Wages D, VanDoren S, Corash L. Photochemically treated fresh frozen plasma for transfusion of patients with acquired co-agulopathy of liver disease. Blood. 2006;107:3753–3760. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

