Amino acids and insulin are regulators of muscle protein synthesis in neonatal pigs
- PMID: 21779306
- PMCID: PMC3139366
- DOI: 10.1017/S1751731110000984
Amino acids and insulin are regulators of muscle protein synthesis in neonatal pigs
Abstract
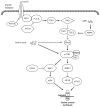
The stage of development between birth and weaning in mammals is a period of very rapid growth that is crucial for the long-term well-being of the animal. The rate of protein deposition in neonatal animals is very high because dietary protein is efficiently utilized to increase body protein mass. Our studies in neonatal pigs have shown that this high efficiency of protein deposition is largely due to the marked increase in protein synthesis after feeding, and this response is particularly profound in the skeletal muscle. The enhanced stimulation of muscle protein synthesis in neonates after feeding is independently mediated by the rise in insulin and amino acids and this response declines with age. Intracellular signaling components that respond to the postprandial rise in amino acids and insulin have been identified and their activation has been shown to be elevated in skeletal muscle of neonatal pigs after a meal and to decrease with development. The enhanced activation of these components in the amino acid and insulin signaling pathways in neonatal muscle contributes to the high rate of muscle protein synthesis and rapid gain in skeletal muscle mass in newborn pigs, which are essential determinants of efficient growth during development.
Figures
References
-
- Avruch J, Lin Y, Long X, Murthy S, Ortiz-Vega S. Recent advances in the regulation of the TOR pathway by insulin and amino acids. Current Opinion in Clinical Nutrition and Metabolic Care. 2005;8:67–72. - PubMed
-
- Baillie AG, Garlick PJ. Attenuated responses of muscle protein synthesis to fasting and insulin in adult female rats. American Journal of Physiology. 1992;262:E1–E5. - PubMed
-
- Bennet WM, Connacher AA, Scrimgeour CM, Rennie MJ. The effect of amino acid infusion on leg protein turnover assessed by L-[15N]phenylalanine and L-[1-13C]leucine exchange. European Journal of Clinical Investigation. 1990;20:41–50. - PubMed
-
- Bevan P. Insulin signalling. Journal of Cell Science. 2001;114:1429–1430. - PubMed
-
- Burrin DG, Davis TA, Ebner S, Schoknecht PA, Fiorotto ML, Reeds PJ, McAvoy S. Nutrient-independent and nutrient-dependent factors stimulate protein synthesis in colostrum-fed newborn pigs. Pediatric Research. 1995;37:593–599. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
