Reduced cAMP, Akt activation and p65-c-Rel dimerization: mechanisms involved in the protective effects of mGluR3 agonists in cultured astrocytes
- PMID: 21779400
- PMCID: PMC3136520
- DOI: 10.1371/journal.pone.0022235
Reduced cAMP, Akt activation and p65-c-Rel dimerization: mechanisms involved in the protective effects of mGluR3 agonists in cultured astrocytes
Abstract
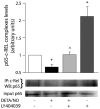
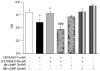
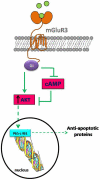
In recent decades, astrocytes have emerged as key pieces in the maintenance of normal functioning of the central nervous system. Any impairment in astroglial function can ultimately lead to generalized disturbance in the brain, thus pharmacological targets associated with prevention of astrocyte death are actually promising. Subtype 3 of metabotropic glutamate receptors (mGluR3) is present in astrocytes, its activation exerting neuroprotective roles. In fact, we have previously demonstrated that mGluR3 selective agonists prevent nitric oxide (NO)-induced astrocyte death. However, mechanisms responsible for that cytoprotective property are still subject to study. Although inhibition of adenylyl cyclase by mGluR3 activation was extensively reported, the involvement of reduced cAMP levels in the effects of mGluR3 agonists and the association between cAMP decrease and the downstream pathways activated by mGluR3 remain neglected. Thus, we studied intracellular signaling mediating anti-apoptotic actions of mGluR3 in cultured rat astrocytes exposed to NO. In the present work, we showed that the cytoprotective effect of mGluR3 agonists (LY379268 and LY404039) requires both the reduction of intracellular cAMP levels and activation of Akt, as assessed by MTT and TUNEL techniques. Moreover, dibutyryl-cAMP impairs Akt phosphorylation induced by LY404039, indicating a relationship between mGluR3-reduced cAMP levels and PI3K/Akt pathway activation. We also demonstrated, by co-immunoprecipitation followed by western-blot, that the mGluR3 agonists not only induce per se survival-linked interaction between members of the NF-κB family p65 and c-Rel, but also impede reduction of levels of p65-c-Rel dimers caused by NO, suggesting a possible anti-apoptotic role for p65-c-Rel. All together, these data suggest that mGluR3 agonists may regulate cAMP/Akt/p65-c-Rel pathway, which would contribute to the protective effect of mGluR3 against NO challenge in astrocytes. Our results widen the knowledge about mechanisms of action of mGluR3, potential targets for the treatment of neurodegenerative disorders where a pathophysiological role for NO has been established.
Conflict of interest statement
Figures





References
-
- Benarroch EE. Neuron-astrocyte interactions: partnership for normal function and disease in the central nervous system. Mayo Clin Proc. 2005;80:1326–1338. - PubMed
-
- Caruso C, Durand D, Schiöth HB, Rey R, Seilicovich A, et al. Activation of melanocortin 4 receptor reduces the inflammatory response and prevents apoptosis induced by lipopolisaccharide and interferon-gamma in astrocytes. Endocrinology. 2007;148:4918–4926. - PubMed
-
- Durand D, Caruso C, Carniglia L, Lasaga M. Metabotropic glutamate receptor 3 activation prevents nitric oxide-induced death in cultured rat astrocytes. J Neurochem. 2010;112:420–433. - PubMed
-
- Schoepp DD, Jane DE, Monn JA. Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology. 1999;38:1431–76. - PubMed
-
- Mudo G, Trovato-Salinaro A, Caniglia G, Cheng Q, Condorelli DF. Cellular localization of mGluR3 and mGluR5 mRNAs in normal and injured rat brain. Brain Res. 2007;1149:1–13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

