Upregulation of Poly (ADP-Ribose) Polymerase-1 (PARP1) in Triple-Negative Breast Cancer and Other Primary Human Tumor Types
- PMID: 21779467
- PMCID: PMC3092251
- DOI: 10.1177/1947601910383418
Upregulation of Poly (ADP-Ribose) Polymerase-1 (PARP1) in Triple-Negative Breast Cancer and Other Primary Human Tumor Types
Abstract
Poly (ADP-ribose) polymerase-1 (PARP1) is a key facilitator of DNA repair and is implicated in pathways of tumorigenesis. PARP inhibitors have gained recent attention as rationally designed therapeutics for the treatment of several malignancies, particularly those associated with dysfunctional DNA repair pathways, including triple-negative breast cancer (TNBC). We investigated the PARP1 gene expression profile in surgical samples from more than 8,000 primary malignant and normal human tissues. PARP1 expression was found to be significantly increased in several malignant tissues, including those isolated from patients with breast, uterine, lung, ovarian, and skin cancers, and non-Hodgkin's lymphoma. Within breast infiltrating ductal carcinoma (IDC) samples tested, mean PARP1 expression was significantly higher relative to normal breast tissue, with over 30% of IDC samples demonstrating upregulation of PARP1, compared with 2.9% of normal tissues. Because of known DNA repair defects, including BRCA1 dysfunction, associated with TNBC, exploration of PARP1 expression in breast cancers related to expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) led to the observation that negative expression of any of the 3 receptors was associated with upregulation of PARP1 expression, compared with receptor-positive tissues. To validate these observations, an independent set of breast adenocarcinomas was evaluated and demonstrated >2-fold upregulation of PARP1 in approximately 70% of primary breast adenocarcinomas, including TNBC, compared with syngeneic nonmalignant breast tissues. Immunohistochemistry (IHC) showed that upregulation of the PARP1 gene was consistent with increased protein expression in TNBC. These analyses suggest a potential biological role for PARP1 in several distinct malignancies, including TNBC. Further investigation of PARP1 as a biomarker for the therapeutic activity of PARP inhibitor-based therapy is warranted.
Keywords: BRCA; PARP; cancer; targeted therapies; triple-negative breast cancer.
Conflict of interest statement
Valeria Ossovskaya, Ingrid Koo, and Barry Sherman are employees of BiPar Sciences Inc. Eric Kaldjian and Christopher Alvares are former employees of Gene Logic Inc.
Figures



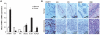
References
-
- Kim MY, Zhang T, Kraus WL. Poly(ADP-ribosyl)ation by PARP-1: ‘PAR-laying’ NAD+ into a nuclear signal. Genes Dev. 2005;19:1951-67 - PubMed
-
- Burkle A. Poly(APD-ribosyl)ation, a DNA damage-driven protein modification and regulator of genomic instability. Cancer Lett. 2001;163:1-5 - PubMed
-
- Schreiber V, Dantzer F, Ame JC, de Murcia G. Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol. 2006;7:517-28 - PubMed
-
- Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913-7 - PubMed
-
- Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917-21 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
