The ERK Cascade: Distinct Functions within Various Subcellular Organelles
- PMID: 21779493
- PMCID: PMC3128630
- DOI: 10.1177/1947601911407328
The ERK Cascade: Distinct Functions within Various Subcellular Organelles
Abstract
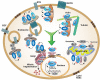
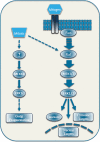
The extracellular signal-regulated kinase 1/2 (ERK1/2) cascade is a central signaling pathway that regulates a wide variety of stimulated cellular processes, including mainly proliferation, differentiation, and survival, but apoptosis and stress response as well. The ability of this linear cascade to induce so many distinct and even opposing effects after various stimulations raises the question as to how the signaling specificity of the cascade is regulated. Over the past years, several specificity-mediating mechanisms have been elucidated, including temporal regulation, scaffolding interactions, crosstalks with other signaling components, substrate competition, and multiple components in each tier of the cascade. In addition, spatial regulation of various components of the cascade is probably one of the main ways by which signals can be directed to some downstream targets and not to others. In this review, we describe first the components of the ERK1/2 cascade and their mode of regulation by kinases, phosphatases, and scaffold proteins. In the second part, we focus on the role of MEK1/2 and ERK1/2 compartmentalization in the nucleus, mitochondria, endosomes, plasma membrane, cytoskeleton, and Golgi apparatus. We explain that this spatial distribution may direct ERK1/2 signals to regulate the organelles' activities. However, it can also direct the activity of the cascade's components to the outer surface of the organelles in order to bring them to close proximity to specific cytoplasmic targets. We conclude that the dynamic localization of the ERK1/2 cascade components is an important regulatory mechanism in determining the signaling specificity of the cascade, and its understanding should shed a new light on the understanding of many stimulus-dependent processes.
Keywords: ERK; Golgi; MAPK; MEK; mitochondria; nucleus.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
Figures
References
-
- Sturgill TW, Ray LB, Erikson E, Maller JL. Insulin-stimulated MAP-2 kinase phosphorylates and activates ribosomal protein S6 kinase II. Nature. 1988;334(6184):715-8 - PubMed
-
- Boulton TG, Nye SH, Robbins DJ, et al. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991;65(4):663-75 - PubMed
-
- Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9(9):726-35 - PubMed
-
- Hibi M, Lin A, Smeal T, Minden A, Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993;7(11):2135-48 - PubMed
-
- Kyriakis JM, Banerjee P, Nikolakaki E, et al. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369(6476):156-60 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
