A critical role for regulatory T cells in driving cytokine profiles of Th17 cells and their modulation of glioma microenvironment
- PMID: 21779877
- PMCID: PMC11028703
- DOI: 10.1007/s00262-011-1069-4
A critical role for regulatory T cells in driving cytokine profiles of Th17 cells and their modulation of glioma microenvironment
Abstract
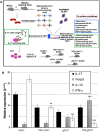
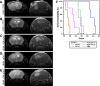
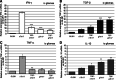
IL-17A, produced by Th17 cells, may play a dual role in antitumor immunity. Using the GL261-glioma model, we investigated the effects of Th17 cells on tumor growth and microenvironment. Th17 cells infiltrate mouse gliomas, increase significantly in a time-dependent manner similarly to Treg and do not express Foxp3. To characterize the direct effects of Th17 cells on GL261 murine gliomas and on tumor microenvironment, we isolated IL-17-producing cells enriched from splenocytes derived from naïve (nTh17) or glioma-bearing mice (gTh17) and pre-stimulated in vitro with or without TGF-β. Spleen-derived Th17 cells co-expressing IL-17, IFN-γ and IL-10, but not Treg marker Foxp3, were co-injected intracranially with GL261 in immune-competent mice. Mice co-injected with GL261 and nTh17 survived significantly longer than gTh17 (P < 0.006) and gliomas expressed high level of IFN-γ and TNF-α, low levels of IL-10 and TGF-β. In vitro IL-17 per se did not exert effects on GL261 proliferation; in vivo gliomas grew equally well intracranially in IL-17 deficient and wild-type mice. We further analyzed relationship between Th17 cells and Treg. Treg were significantly higher in splenocytes from glioma-bearing than naïve mice (P = 0.01) and gTh17 produced more IL-10 than IFN-γ (P = 0.002). In vitro depletion of Treg using PC61 in splenocytes from glioma-bearing mice causes increased IL-17/IFN-γ cells (P = 0.007) and decreased IL-17/IL-10 cells (P = 0.03). These results suggest that Th17 polarization may be induced by Treg and that Th17 cells in gliomas modulate tumor growth depending on locally produced cytokines.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

