Constitutive expression of short hairpin RNA in vivo triggers buildup of mature hairpin molecules
- PMID: 21780944
- PMCID: PMC3237692
- DOI: 10.1089/hum.2010.234
Constitutive expression of short hairpin RNA in vivo triggers buildup of mature hairpin molecules
Abstract
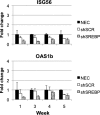
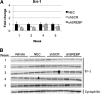
RNA interference (RNAi) has become the cornerstone technology for studying gene function in mammalian cells. In addition, it is a promising therapeutic treatment for multiple human diseases. Virus-mediated constitutive expression of short hairpin RNA (shRNA) has the potential to provide a permanent source of silencing molecules to tissues, and it is being devised as a strategy for the treatment of liver conditions such as hepatitis B and hepatitis C virus infection. Unintended interaction between silencing molecules and cellular components, leading to toxic effects, has been described in vitro. Despite the enormous interest in using the RNAi technology for in vivo applications, little is known about the safety of constitutively expressing shRNA for multiple weeks. Here we report the effects of in vivo shRNA expression, using helper-dependent adenoviral vectors. We show that gene-specific knockdown is maintained for at least 6 weeks after injection of 1 × 10(11) viral particles. Nonetheless, accumulation of mature shRNA molecules was observed up to weeks 3 and 4, and then declined gradually, suggesting the buildup of mature shRNA molecules induced cell death with concomitant loss of viral DNA and shRNA expression. No evidence of well-characterized innate immunity activation (such as interferon production) or saturation of the exportin-5 pathway was observed. Overall, our data suggest constitutive expression of shRNA results in accumulation of mature shRNA molecules, inducing cellular toxicity at late time points, despite the presence of gene silencing.
Figures




References
-
- Banerjee S. Neveu P. Kosik K.S. A coordinated local translational control point at the synapse involving relief from silencing and MOV10 degradation. Neuron. 2009;64:871–884. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

