A monoclonal antibody against synthetic Aβ dimer assemblies neutralizes brain-derived synaptic plasticity-disrupting Aβ
- PMID: 21781116
- PMCID: PMC3174526
- DOI: 10.1111/j.1471-4159.2011.07389.x
A monoclonal antibody against synthetic Aβ dimer assemblies neutralizes brain-derived synaptic plasticity-disrupting Aβ
Abstract
Diverse lines of evidence indicate that pre-fibrillar, diffusible assemblies of the amyloid β-protein (Aβ) play an important role in Alzheimer's disease pathogenesis. Although the precise molecular identity of these soluble toxins remains unsettled, recent experiments suggest that sodium dodecyl sulfate (SDS)-stable Aβ dimers may be the basic building blocks of Alzheimer's disease-associated synaptotoxic assemblies and as such present an attractive target for therapeutic intervention. In the absence of sufficient amounts of highly pure cerebral Aβ dimers, we have used synthetic disulfide cross-linked dimers (free of Aβ monomer or fibrils) to generate conformation-specific monoclonal antibodies. These dimers aggregate to form kinetically trapped protofibrils, but do not readily form fibrils. We identified two antibodies, 3C6 and 4B5, which preferentially bind assemblies formed from covalent Aβ dimers, but do not bind to Aβ monomer, amyloid precursor protein, or aggregates formed by other amyloidogenic proteins. Monoclonal antibody 3C6, but not an IgM isotype-matched control antibody, ameliorated the plasticity-disrupting effects of Aβ extracted from the aqueous phase of Alzheimer's disease brain, thus suggesting that 3C6 targets pathogenically relevant Aβ assemblies. These data prove the usefulness of covalent dimers and their assemblies as immunogens and recommend further investigation of the therapeutic and diagnostic utility of monoclonal antibodies raised to such assemblies.
© 2011 The Authors. Journal of Neurochemistry © 2011 International Society for Neurochemistry.
Conflict of interest statement
Figures


 ) and Aβ1-40S26C (
) and Aβ1-40S26C ( ) samples on a Superdex™ 75 10/300-GL column eluted with 25 mM ammonium acetate, pH 8.5, confirmed their high purity and the absence of higher molecular weight Aβ assemblies. (F) Antibody binding curves for purified IgMκ's 3C6 (■, □) and 4B5 (▲,Δ), and control mAb 6E10 (♦, ◊) against plate-immobilized freshly isolated (Aβ1-40S26C)2 (closed symbols) and Aβ1-40S26C (open symbols). Antibody binding curves were also determined for 3C6 (G) and 4B5 (H) binding to: freshly-isolated (Aβ1-40S26C)2 (●); (Aβ1-40S26C)2 stored for ~1 month at −80 °C in 25 mM ammonium acetate, pH 8.5, (▼); Freshly-isolated Aβ1-40S26C monomers (□), and WT Aβ1-40 fibrils (◊). Antibody binding studies were carried out in PBSA containing 1% BSA and 0.05% Tween 20, pH 7.4.
) samples on a Superdex™ 75 10/300-GL column eluted with 25 mM ammonium acetate, pH 8.5, confirmed their high purity and the absence of higher molecular weight Aβ assemblies. (F) Antibody binding curves for purified IgMκ's 3C6 (■, □) and 4B5 (▲,Δ), and control mAb 6E10 (♦, ◊) against plate-immobilized freshly isolated (Aβ1-40S26C)2 (closed symbols) and Aβ1-40S26C (open symbols). Antibody binding curves were also determined for 3C6 (G) and 4B5 (H) binding to: freshly-isolated (Aβ1-40S26C)2 (●); (Aβ1-40S26C)2 stored for ~1 month at −80 °C in 25 mM ammonium acetate, pH 8.5, (▼); Freshly-isolated Aβ1-40S26C monomers (□), and WT Aβ1-40 fibrils (◊). Antibody binding studies were carried out in PBSA containing 1% BSA and 0.05% Tween 20, pH 7.4.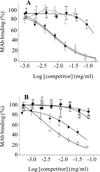
References
-
- Alzheimer A. Über einen eigenartigen schweren Erkrankungsproze! der Hirnrinde. Neurologisches Centralblatt. 1906;23:1129–1136.
-
- Arancio O, Chao MV. Neurotrophins, synaptic plasticity and dementia. Curr Opin Neurobiol. 2007;17:325–330. - PubMed
-
- Barghorn S, Nimmrich V, Striebinger A, Krantz C, Keller P, Janson B, Bahr M, Schmidt M, Bitner RS, Harlan J, Barlow E, Ebert U, Hillen H. Globular amyloid beta-peptide oligomer - a homogenous and stable neuropathological protein in Alzheimer's disease. J Neurochem. 2005;95:834–847. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

