Role of stem cells in cardiovascular biology
- PMID: 21781250
- PMCID: PMC4071762
- DOI: 10.1111/j.1538-7836.2011.04363.x
Role of stem cells in cardiovascular biology
Abstract
This review article addresses the controversy as to whether the adult heart possesses an intrinsic growth reserve. If myocyte renewal takes place in healthy and diseased organs, the reconstitution of the damaged tissue lost upon pathological insults might be achieved by enhancing a natural occurring process. Evidence in support of the old and new view of cardiac biology is critically discussed in an attempt to understand whether the heart is a static or dynamic organ. According to the traditional concept, the heart exerts its function until death of the organism with the same or lesser number of cells that are present at birth. This paradigm was challenged by documentation of the cell cycle activation and nuclear and cellular division in a subset of myocytes. These observations raised the important question of the origin of replicating myocytes. Several theories have been proposed and are presented in this review article. Newly formed myocytes may derive from a pre-existing pool of cells that has maintained the ability to divide. Alternatively, myocytes may be generated by activation and commitment of resident cardiac stem cells or by migration of progenitor cells from distant organs. In all cases, parenchymal cell turnover throughout lifespan results in a heterogeneous population consisting of young, adult, and senescent myocytes. With time, accumulation of old myocytes has detrimental effects on cardiac performance and may cause the development of an aging myopathy.
© 2011 International Society on Thrombosis and Haemostasis.
Conflict of interest statement
The authors state that they have no conflict of interest.
Figures
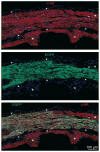
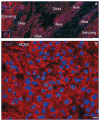
References
-
- Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D’Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, et al. Human cardiac stem cells. Proc Natl Acad Sci USA. 2007;104:14068–73. - PMC - PubMed
-
- Hosoda T, D’Amario D, Cabral-Da-Silva MC, Zheng H, Padin-Iruegas ME, Ogorek B, Ferreira-Martins J, Yasuzawa-Amano S, Amano K, Ide-Iwata N, Cheng W, Rota M, Urbanek K, Kajstura J, Anversa P, Leri A. Clonality of mouse and human cardiomyogenesis in vivo. Proc Natl Acad Sci USA. 2009;106:17169–74. - PMC - PubMed
-
- Taylor DA, Atkins BZ, Hungspreugs P, Jones TR, Reedy MC, Hutcheson KA, Glower DD, Kraus WE. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat Med. 1998;4:929–33. - PubMed
-
- Galli D, Innocenzi A, Staszewsky L, Zanetta L, Sampaolesi M, Bai A, Martinoli E, Carlo E, Balconi G, Fiordaliso F, Chimenti S, Cusella G, Dejana E, Cossu G, Latini R. Mesoangioblasts, vessel-associated multipotent stem cells, repair the infarcted heart by multiple cellular mechanisms: a comparison with bone marrow progenitors, fibroblasts, and endothelial cells. Arterioscler Thromb Vasc Biol. 2005;25:692–7. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

