Characterization of chronic HCV infection-induced apoptosis
- PMID: 21781333
- PMCID: PMC3160349
- DOI: 10.1186/1476-5926-10-4
Characterization of chronic HCV infection-induced apoptosis
Abstract
Background: To understand the complex and largely not well-understood apoptotic pathway and immune system evasion mechanisms in hepatitis C virus (HCV)-associated hepatocellular carcinoma (HCC) and HCV associated chronic hepatitis (CH), we studied the expression patterns of a number of pro-apoptotic and anti-apoptotic genes (Fas, FasL, Bcl-2, Bcl-xL and Bak) in HepG2 cell line harboring HCV- genotype-4 replication. For confirmation, we also assessed the expression levels of the same group of genes in clinical samples obtained from 35 HCC and 34 CH patients.
Methods: Viral replication was assessed in the tissue culture medium by RT-PCR, quantitative Real-Time PCR (qRT-PCR); detection of HCV core protein by western blot and inhibition of HCV replication with siRNA. The expression level of Fas, FasL, Bcl-2, Bcl-xL and Bak was assessed by immunohistochemistry and RT-PCR whereas caspases 3, 8 and 9 were assessed by colorimetric assay kits up to 135 days post infection.
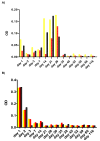
Results: There was a consistent increase in apoptotic activity for the first 4 weeks post-CV infection followed by a consistent decrease up to the end of the experiment. The concordance between the changes in the expression levels of Fas, FasL, Bcl-2, Bcl-xL and Bak in vitro and in situ was statistically significant (p < 0.05). Fas was highly expressed at early stages of infection in cell lines and in normal control liver tissues followed by a dramatic reduction post-HCV infection and an increase in the expression level of FasL post HCV infection. The effect of HCV infection on other apoptotic proteins started very early post-infection, suggesting that hepatitis C modulating apoptosis by modulating intracellular pro-apoptotic signals.
Conclusions: Chronic HCV infection differently modulates the apoptotic machinery during the course of infection, where the virus induces apoptosis early in the course of infection, and as the disease progresses apoptosis is modulated. This study could open a new opportunity for understanding the various signaling of apoptosis and in the developing a targeted therapy to inhibit viral persistence and HCC development.
Figures





References
-
- Eassa S, Eissa M, Sharaf SM, Ibrahim MH, Hassanein OM. Prevalence of hepatitis C virus infection and evaluation of a health education program in el-ghar village in zagazig, egypt. J Egypt Public Health Assoc. 2007;82(5-6):379–404. - PubMed
-
- El-Karaksy HM, Anwar G, Esmat G, Mansour S, Sabry M, Helmy H, El-Hennawy A, Fouad H. Prevalence of hepatic abnormalities in a cohort of Egyptian children with type 1 diabetes mellitus. Pediatr Diabetes. 2009;1(7):462–70. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
