Electron transport in acetate-grown Methanosarcina acetivorans
- PMID: 21781343
- PMCID: PMC3160891
- DOI: 10.1186/1471-2180-11-165
Electron transport in acetate-grown Methanosarcina acetivorans
Abstract
Background: Acetate is the major source of methane in nature. The majority of investigations have focused on acetotrophic methanogens for which energy-conserving electron transport is dependent on the production and consumption of H₂ as an intermediate, although the great majority of acetotrophs are unable to metabolize H₂. The presence of cytochrome c and a complex (Ma-Rnf) homologous to the Rnf (Rhodobacter nitrogen fixation) complexes distributed in the domain Bacteria distinguishes non-H₂-utilizing Methanosarcina acetivorans from H₂-utilizing species suggesting fundamentally different electron transport pathways. Thus, the membrane-bound electron transport chain of acetate-grown M. acetivorans was investigated to advance a more complete understanding of acetotrophic methanogens.
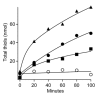
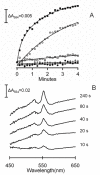
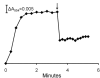
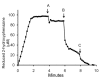
Results: A component of the CO dehydrogenase/acetyl-CoA synthase (CdhAE) was partially purified and shown to reduce a ferredoxin purified using an assay coupling reduction of the ferredoxin to oxidation of CdhAE. Mass spectrometry analysis of the ferredoxin identified the encoding gene among annotations for nine ferredoxins encoded in the genome. Reduction of purified membranes from acetate-grown cells with ferredoxin lead to reduction of membrane-associated multi-heme cytochrome c that was re-oxidized by the addition of either the heterodisulfide of coenzyme M and coenzyme B (CoM-S-S-CoB) or 2-hydoxyphenazine, the soluble analog of methanophenazine (MP). Reduced 2-hydoxyphenazine was re-oxidized by membranes that was dependent on addition of CoM-S-S-CoB. A genomic analysis of Methanosarcina thermophila, a non-H2-utilizing acetotrophic methanogen, identified genes homologous to cytochrome c and the Ma-Rnf complex of M. acetivorans.
Conclusions: The results support roles for ferredoxin, cytochrome c and MP in the energy-conserving electron transport pathway of non-H₂-utilizing acetotrophic methanogens. This is the first report of involvement of a cytochrome c in acetotrophic methanogenesis. The results suggest that diverse acetotrophic Methanosarcina species have evolved diverse membrane-bound electron transport pathways leading from ferredoxin and culminating with MP donating electrons to the heterodisulfide reductase (HdrDE) for reduction of CoM-S-S-CoB.
Figures


Similar articles
-
Characterization of a CO: heterodisulfide oxidoreductase system from acetate-grown Methanosarcina thermophila.J Bacteriol. 1994 Nov;176(22):6974-9. doi: 10.1128/jb.176.22.6974-6979.1994. J Bacteriol. 1994. PMID: 7961460 Free PMC article.
-
A Membrane-Bound Cytochrome Enables Methanosarcina acetivorans To Conserve Energy from Extracellular Electron Transfer.mBio. 2019 Aug 20;10(4):e00789-19. doi: 10.1128/mBio.00789-19. mBio. 2019. PMID: 31431545 Free PMC article.
-
A Ferredoxin- and F420H2-Dependent, Electron-Bifurcating, Heterodisulfide Reductase with Homologs in the Domains Bacteria and Archaea.mBio. 2017 Feb 7;8(1):e02285-16. doi: 10.1128/mBio.02285-16. mBio. 2017. PMID: 28174314 Free PMC article.
-
Bioenergetics and anaerobic respiratory chains of aceticlastic methanogens.Biochim Biophys Acta. 2014 Jul;1837(7):1130-47. doi: 10.1016/j.bbabio.2013.12.002. Epub 2013 Dec 12. Biochim Biophys Acta. 2014. PMID: 24333786 Review.
-
Electron Bifurcation and Confurcation in Methanogenesis and Reverse Methanogenesis.Front Microbiol. 2018 Jun 20;9:1322. doi: 10.3389/fmicb.2018.01322. eCollection 2018. Front Microbiol. 2018. PMID: 29973922 Free PMC article. Review.
Cited by
-
Comparative genomics reveals electron transfer and syntrophic mechanisms differentiating methanotrophic and methanogenic archaea.PLoS Biol. 2022 Jan 5;20(1):e3001508. doi: 10.1371/journal.pbio.3001508. eCollection 2022 Jan. PLoS Biol. 2022. PMID: 34986141 Free PMC article. Review.
-
Assessing the Ecophysiology of Methanogens in the Context of Recent Astrobiological and Planetological Studies.Life (Basel). 2015 Dec 3;5(4):1652-86. doi: 10.3390/life5041652. Life (Basel). 2015. PMID: 26703739 Free PMC article. Review.
-
In vivo activation of methyl-coenzyme M reductase by carbon monoxide.Front Microbiol. 2013 Apr 1;4:69. doi: 10.3389/fmicb.2013.00069. eCollection 2013. Front Microbiol. 2013. PMID: 23554601 Free PMC article.
-
Reversing methanogenesis to capture methane for liquid biofuel precursors.Microb Cell Fact. 2016 Jan 14;15:11. doi: 10.1186/s12934-015-0397-z. Microb Cell Fact. 2016. PMID: 26767617 Free PMC article.
-
Mechanisms for Electron Uptake by Methanosarcina acetivorans during Direct Interspecies Electron Transfer.mBio. 2021 Oct 26;12(5):e0234421. doi: 10.1128/mBio.02344-21. Epub 2021 Oct 5. mBio. 2021. PMID: 34607451 Free PMC article.
References
-
- Meuer J, Kuettner HC, Zhang JK, Hedderich R, Metcalf WW. Genetic analysis of the archaeon Methanosarcina barkeri Fusaro reveals a central role for Ech hydrogenase and ferredoxin in methanogenesis and carbon fixation. Proc Natl Acad Sci USA. 2002;99(8):5632–5637. doi: 10.1073/pnas.072615499. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources

