Mn bioavailability by polarized Caco-2 cells: comparison between Mn gluconate and Mn oxyprolinate
- PMID: 21781350
- PMCID: PMC3171306
- DOI: 10.1186/1475-2891-10-77
Mn bioavailability by polarized Caco-2 cells: comparison between Mn gluconate and Mn oxyprolinate
Abstract
Background: Micronutrient inadequate intake is responsible of pathological deficiencies and there is a need of assessing the effectiveness of metal supplementation, frequently proposed to rebalance poor diets. Manganese (Mn) is present in many enzymatic intracellular systems crucial for the regulation of cell metabolism, and is contained in commercially available metal supplements.
Methods: We compared the effects of two different commercial Mn forms, gluconate (MnGluc) and oxyprolinate (MnOxP). For this purpose we used the polarized Caco-2 cells cultured on transwell filters, an established in vitro model of intestinal epithelium. Since micronutrient deficiency may accelerate mitochondrial efficiency, the mitochondrial response of these cells, in the presence of MnGluc and MnOxP, by microscopy methods and by ATP luminescence assay was used.
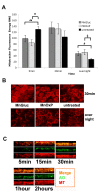
Results: In the presence of both MnOxP and MnGluc a sustained mitochondrial activity was shown by mitoTraker labeling (indicative of mitochondrial respiration), but ATP intracellular content remained comparable to untreated cells only in the presence of MnOxP. In addition MnOxP transiently up-regulated the antioxidant enzyme Mn superoxide dismutase more efficiently than MnGluc. Both metal treatments preserved NADH and βNADPH diaphorase oxidative activity, avoided mitochondrial dysfunction, as assessed by the absence of a sustained phosphoERK activation, and were able to maintain cell viability.
Conclusions: Collectively, our data indicate that MnOxP and MnGluc, and primarily the former, produce a moderate and safe modification of Caco-2 cell metabolism, by activating positive enzymatic mechanisms, thus could contribute to long-term maintenance of cell homeostasis.
Figures




Similar articles
-
[Bioavailabilities of manganese sources based on heart manganese-containing superoxide dismutase gene expression for broilers].Wei Sheng Yan Jiu. 2004 Nov;33(6):681-6. Wei Sheng Yan Jiu. 2004. PMID: 15727177 Chinese.
-
Human intestinal Caco-2 cell line in vitro assay to evaluate the absorption of Cd, Cu, Mn and Zn from urban environmental matrices.Environ Geochem Health. 2020 Feb;42(2):601-615. doi: 10.1007/s10653-019-00394-4. Epub 2019 Aug 19. Environ Geochem Health. 2020. PMID: 31428946
-
Dietary manganese source does not affect Mn, Zn and Cu tissue deposition and the activity of manganese-containing enzymes in lambs.J Trace Elem Med Biol. 2016 Dec;38:138-143. doi: 10.1016/j.jtemb.2016.05.003. Epub 2016 May 20. J Trace Elem Med Biol. 2016. PMID: 27267351
-
Methods and options for estimating iron and zinc bioavailability using Caco-2 cell models: benefits and limitations.Int J Vitam Nutr Res. 2005 Nov;75(6):413-21. doi: 10.1024/0300-9831.75.6.413. Int J Vitam Nutr Res. 2005. PMID: 16711475 Review.
-
Manganese Superoxide Dismutase Dysfunction and the Pathogenesis of Kidney Disease.Front Physiol. 2020 Jul 14;11:755. doi: 10.3389/fphys.2020.00755. eCollection 2020. Front Physiol. 2020. PMID: 32760286 Free PMC article. Review.
Cited by
-
Bioavailability Assessment of an Iron Formulation Using Differentiated Human Intestinal Caco-2 Cells.Foods. 2023 Aug 11;12(16):3016. doi: 10.3390/foods12163016. Foods. 2023. PMID: 37628015 Free PMC article.
-
Mitochondrial Energetics and Ca2+-Activated ATPase in Obstructive Hypertrophic Cardiomyopathy.J Clin Med. 2020 Jun 9;9(6):1799. doi: 10.3390/jcm9061799. J Clin Med. 2020. PMID: 32527005 Free PMC article.
References
-
- Shankar AH, Prasad AS. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr. 1998;68(2 Suppl):447S–463S. - PubMed
-
- Prasad AS. Zinc: mechanisms of host defense. J Nutr. 2007;137(5):1345–1349. - PubMed
-
- Munoz C, Rios E, Olivos J, Brunser O, Olivares M. Iron, copper and immunocompetence. Br J Nutr. 2007;98(Suppl 1):S24–28. - PubMed

