Sesquiterpene lactones from Gynoxys verrucosa and their anti-MRSA activity
- PMID: 21782013
- PMCID: PMC3159821
- DOI: 10.1016/j.jep.2011.07.012
Sesquiterpene lactones from Gynoxys verrucosa and their anti-MRSA activity
Erratum in
-
Corrigendum to "Sesquiterpene lactones from Gynoxys verrucosa and their anti-MRSA activity" [J. Ethnopharmacol. 137 (2011) 1055-1059].J Ethnopharmacol. 2016 Jun 20;186:392. doi: 10.1016/j.jep.2016.04.022. Epub 2016 May 8. J Ethnopharmacol. 2016. PMID: 27165010 No abstract available.
Abstract
Ethnopharmacological relevance: Because of its virulence and antibiotic resistance, Staphylococcus aureus is a more formidable pathogen now than at any time since the pre-antibiotic era. In an effort to identify and develop novel antimicrobial agents with activity against this pathogen, we have examined Gynoxys verrucosa Wedd (Asteraceae), an herb used in traditional medicine in southern Ecuador for the treatment and healing of wounds.
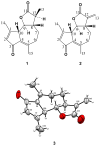
Materials and methods: The sesquiterpene lactones leucodine (1) and dehydroleucodine (2) were extracted and purified from the aerial parts of Gynoxys verrucosa, and their structure was elucidated by spectroscopic methods and single-crystal X-ray analysis. The in vitro anti-microbial activity of Gynoxys verrucosa extracts and its purified constituents was determined against six clinical isolates including Staphylococcus aureus and Staphylococcus epidermidis strains with different drug-resistance profiles, using the microtiter broth method.
Results: Compound 1 has very low activity, while compound 2 has moderate activity with MIC(50)s between 49 and 195 μg/mL. The extract of Gynoxys verrucosa has weak activity with MIC(50)s between 908 and 3290 μg/mL.
Conclusions: We are reporting the full assignment of the (1)H NMR and (13)C NMR of both compounds, and the crystal structure of compound 2, for the first time. Moreover, the fact that compound 2 has antimicrobial activity and compound 1 does not, demonstrates that the exocyclic conjugated methylene in the lactone ring is essential for the antimicrobial activity of these sesquiterpene lactones. However, the weak activity observed for the plant extracts, does not explain the use of Gynoxys verrucosa in traditional medicine for the treatment of wounds and skin infections.
Copyright © 2011 Elsevier Ireland Ltd. All rights reserved.
Figures
References
-
- Amsterdam D. Susceptibility testing of antimicrobials in liquid media. In: Loman V, editor. Antibiotics in Laboratory Medicine. 4. Williams and Wilkins; Baltimore, MD: 1996. pp. 52–111.
-
- Deurenberg RH, Stobberingh EE. The molecular evolution of hospital and community associated methicillin-resistant Staphylococcus aureus. Current Molecular Medicine. 2009;9:100–15. - PubMed
-
- Gibbons S. Anti-staphylococcal plant natural products. Natural Products Reports. 2004;21:263–277. - PubMed
-
- Hilmi F, Sticher O, Heilmann J. New cytotoxic sesquiterpene lactones from Warionia saharae. Planta Medica. 2003;69:462–464. - PubMed
-
- Isenberg HD. Clinical Microbiology Procedures Handbook. 2004;2:5.1–5.1.15.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical