Mutations in ANKRD11 cause KBG syndrome, characterized by intellectual disability, skeletal malformations, and macrodontia
- PMID: 21782149
- PMCID: PMC3155157
- DOI: 10.1016/j.ajhg.2011.06.007
Mutations in ANKRD11 cause KBG syndrome, characterized by intellectual disability, skeletal malformations, and macrodontia
Abstract
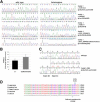
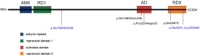
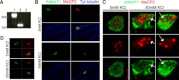
KBG syndrome is characterized by intellectual disability associated with macrodontia of the upper central incisors as well as distinct craniofacial findings, short stature, and skeletal anomalies. Although believed to be genetic in origin, the specific underlying defect is unknown. Through whole-exome sequencing, we identified deleterious heterozygous mutations in ANKRD11 encoding ankyrin repeat domain 11, also known as ankyrin repeat-containing cofactor 1. A splice-site mutation, c.7570-1G>C (p.Glu2524_Lys2525del), cosegregated with the disease in a family with three affected members, whereas in a simplex case a de novo truncating mutation, c.2305delT (p.Ser769GlnfsX8), was detected. Sanger sequencing revealed additional de novo truncating ANKRD11 mutations in three other simplex cases. ANKRD11 is known to interact with nuclear receptor complexes to modify transcriptional activation. We demonstrated that ANKRD11 localizes mainly to the nuclei of neurons and accumulates in discrete inclusions when neurons are depolarized, suggesting that it plays a role in neural plasticity. Our results demonstrate that mutations in ANKRD11 cause KBG syndrome and outline a fundamental role of ANKRD11 in craniofacial, dental, skeletal, and central nervous system development and function.
Copyright © 2011 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Brancati F., D'Avanzo M.G., Digilio M.C., Sarkozy A., Biondi M., De Brasi D., Mingarelli R., Dallapiccola B. KBG syndrome in a cohort of Italian patients. Am. J. Med. Genet. A. 2004;131:144–149. - PubMed
-
- Zollino M., Battaglia A., D'Avanzo M.G., Della Bruna M.M., Marini R., Scarano G., Cappa M., Neri G. Six additional cases of the KBG syndrome: Clinical reports and outline of the diagnostic criteria. Am. J. Med. Genet. 1994;52:302–307. - PubMed
-
- Skjei K.L., Martin M.M., Slavotinek A.M. KBG syndrome: Report of twins, neurological characteristics, and delineation of diagnostic criteria. Am. J. Med. Genet. A. 2007;143:292–300. - PubMed
-
- Herrmann J., Pallister P.D., Tiddy W., Opitz J.M. The KBG syndrome-a syndrome of short stature, characteristic facies, mental retardation, macrodontia and skeletal anomalies. Birth Defects Orig. Artic. Ser. 1975;11:7–18. - PubMed
-
- Tekin M., Kavaz A., Berberoğlu M., Fitoz S., Ekim M., Ocal G., Akar N. The KBG syndrome: Confirmation of autosomal dominant inheritance and further delineation of the phenotype. Am. J. Med. Genet. A. 2004;130A:284–287. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

