Morin attenuates tau hyperphosphorylation by inhibiting GSK3β
- PMID: 21782947
- PMCID: PMC3166962
- DOI: 10.1016/j.nbd.2011.07.005
Morin attenuates tau hyperphosphorylation by inhibiting GSK3β
Abstract
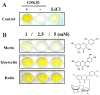
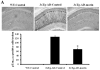
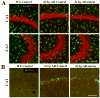
Alzheimer's disease (AD) is the major form of age-related dementia and is characterized by progressive cognitive impairment, the accumulation of extracellular amyloid β-peptide (Aβ), and intracellular hyperphosphorylated tau aggregates in affected brain regions. Tau hyperphosphorylation and accumulation in neurofibrillary tangles is strongly correlated with cognitive deficits, and is apparently a critical event in the dementia process because mutations in tau can cause a tangle-only form of dementia called frontotemporal lobe dementia. Among kinases that phosphorylate tau, glycogen synthase kinase 3β (GSK3β) is strongly implicated in AD pathogenesis. In the present study, we established an ELISA to screen for agents that inhibit GSK3β activity and found that the flavonoid morin effectively inhibited GSK3β activity and blocked GSK3β-induced tau phosphorylation in vitro. In addition, morin attenuated Aβ-induced tau phosphorylation and protected human neuroblastoma cells against Aβ cytotoxicity. Furthermore, treatment of 3xTg-AD mice with morin resulted in reductions in tau hyperphosphorylation and paired helical filament-like immunoreactivity in hippocampal neurons. Morin is a novel inhibitor of GSK3β that can reduce tau pathology in vivo and may have potential as a therapeutic agent in tauopathies.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures







References
-
- Alonso AC, et al. Alzheimer’s disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules. Nat Med. 1996;2:783–7. - PubMed
-
- Alvarez G, et al. Regulation of tau phosphorylation and protection against beta-amyloid-induced neurodegeneration by lithium. Possible implications for Alzheimer’s disease. Bipolar Disord. 2002;4:153–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

