Microglia: a promising target for treating neuropathic and postoperative pain, and morphine tolerance
- PMID: 21783017
- PMCID: PMC3169792
- DOI: 10.1016/S0929-6646(11)60074-0
Microglia: a promising target for treating neuropathic and postoperative pain, and morphine tolerance
Abstract
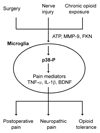
Management of chronic pain, such as nerve-injury-induced neuropathic pain associated with diabetic neuropathy, viral infection, and cancer, is a real clinical challenge. Major surgeries, such as breast and thoracic surgery, leg amputation, and coronary artery bypass surgery, also lead to chronic pain in 10-50% of individuals after acute postoperative pain, partly due to surgery-induced nerve injury. Current treatments mainly focus on blocking neurotransmission in the pain pathway and have only resulted in limited success. Ironically, chronic opioid exposure might lead to paradoxical pain. Development of effective therapeutic strategies requires a better understanding of cellular mechanisms underlying the pathogenesis of neuropathic pain. Progress in pain research points to an important role of microglial cells in the development of chronic pain. Spinal cord microglia are strongly activated after nerve injury, surgical incision, and chronic opioid exposure. Increasing evidence suggests that, under all these conditions, the activated microglia not only exhibit increased expression of microglial markers CD 11 b and Iba 1, but also display elevated phosphorylation of p38 mitogen-activated protein kinase. Inhibition of spinal cord p38 has been shown to attenuate neuropathic and postoperative pain, as well as morphine-induced antinociceptive tolerance. Activation of p38 in spinal microglia results in increased synthesis and release of the neurotrophin brain-derived neurotrophic factor and the proinflammatory cytokines interleukin-1β, interleukin-6, and tumor necrosis factor-α. These microglia-released mediators can powerfully modulate spinal cord synaptic transmission, leading to increased excitability of dorsal horn neurons, that is, central sensitization, partly via suppressing inhibitory synaptic transmission. Here, we review studies that support the pronociceptive role of microglia in conditions of neuropathic and postoperative pain and opioid tolerance. We conclude that targeting microglial signaling might lead to more effective treatments for devastating chronic pain after diabetic neuropathy, viral infection, cancer, and major surgeries, partly via improving the analgesic efficacy of opioids.
Copyright © 2011 Formosan Medical Association & Elsevier. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
All the authors have no financial interests related to the material in the manuscript.
Figures

References
-
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. - PubMed
-
- Echeverry S, Shi XQ, Zhang J. Characterization of cell proliferation in rat spinal cord following peripheral nerve injury and the relationship with neuropathic pain. Pain. 2008;135:37–47. - PubMed
-
- Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

