Posttranslational arginylation as a global biological regulator
- PMID: 21784066
- PMCID: PMC3171647
- DOI: 10.1016/j.ydbio.2011.06.043
Posttranslational arginylation as a global biological regulator
Abstract
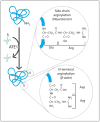
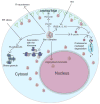
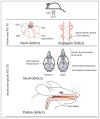
Posttranslational modifications constitute a major field of emerging biological significance as mounting evidence demonstrates their key role in multiple physiological processes. Following in the footsteps of protein phosphorylation studies, new modifications are being shown to regulate protein properties and functions in vivo. Among such modifications, an important role belongs to protein arginylation - posttranslational tRNA-mediated addition of arginine, to proteins by arginyltransferase, ATE1. Recent studies show that arginylation is essential for embryogenesis in many organisms and that it regulates such important processes as heart development, angiogenesis, and tissue morphogenesis in mammals. This review summarizes the key data in the protein arginylation field since its original discovery to date.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

References
-
- Bachmair A, Finley D, Varshavsky A. In vivo half-life of a protein is a function of its amino-terminal residue. Science. 1986;234:179–86. - PubMed
-
- Balzi E, Choder M, Chen WN, Varshavsky A, Goffeau A. Cloning and functional analysis of the arginyl-tRNA-protein transferase gene ATE1 of Saccharomyces cerevisiae. J Biol Chem. 1990;265:7464–71. - PubMed
-
- Berleth ES, Li J, Braunscheidel JA, Pickart CM. A reactive nucleophile proximal to vicinal thiols is an evolutionarily conserved feature in the mechanism of Arg aminoacyl-tRNA protein transferase. Arch Biochem Biophys. 1992;298:498–504. - PubMed
-
- Bohley P, Kopitz J, Adam G. Arginylation, surface hydrophobicity and degradation of cytosol proteins from rat hepatocytes. Adv Exp Med Biol. 1988a;240:159–69. - PubMed
-
- Bohley P, Kopitz J, Adam G. Surface hydrophobicity, arginylation and degradation of cytosol proteins from rat hepatocytes. Biol Chem Hoppe Seyler. 1988b;369(Suppl):307–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

