Protein S-glutathiolation: redox-sensitive regulation of protein function
- PMID: 21784079
- PMCID: PMC3245358
- DOI: 10.1016/j.yjmcc.2011.07.009
Protein S-glutathiolation: redox-sensitive regulation of protein function
Abstract
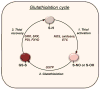
Reversible protein S-glutathiolation has emerged as an important mechanism of post-translational modification. Under basal conditions several proteins remain adducted to glutathione, and physiological glutathiolation of proteins has been shown to regulate protein function. Enzymes that promote glutathiolation (e.g., glutathione-S-transferase-P) or those that remove glutathione from proteins (e.g., glutaredoxin) have been identified. Modification by glutathione has been shown to affect protein catalysis, ligand binding, oligomerization and protein-protein interactions. Conditions associated with oxidative or nitrosative stress, such as ischemia-reperfusion, hypertension and tachycardia increase protein glutathiolation via changes in the glutathione redox status (GSH/GSSG) or through the formation of sulfenic acid (SOH) or nitrosated (SNO) cysteine intermediates. These "activated" thiols promote reversible S-glutathiolation of key proteins involved in cell signaling, energy production, ion transport, and cell death. Hence, S-glutathiolation is ideally suited for integrating and mounting fine-tuned responses to changes in the redox state. S-glutathiolation also provides a temporary glutathione "cap" to protect protein thiols from irreversible oxidation and it could be an important mechanism of protein "encryption" to maintain proteins in a functionally silent state until they are needed during conditions of stress. Current evidence suggests that the glutathiolation-deglutathiolation cycle integrates and interacts with other post-translational mechanisms to regulate signal transduction, metabolism, inflammation, and apoptosis. This article is part of a Special Section entitled "Post-translational Modification."
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures

References
-
- Kaplowitz N, Aw TY, Ookhtens M. The regulation of hepatic glutathione. Annu Rev Pharmacol Toxicol. 1985;25:715–44. - PubMed
-
- Winkler BS, Orselli SM, Rex TS. The redox couple between glutathione and ascorbic acid: a chemical and physiological perspective. Free Radic Biol Med. 1994;17:333–49. - PubMed
-
- Vander Jagt DL, Daub E, Krohn JA, Han LP. Effects of pH and thiols on the kinetics of yeast glyoxalase I. An evaluation of the random pathway mechanism. Biochemistry. 1975;14:3669–75. - PubMed
-
- Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

