Extended results of the Alzheimer's disease anti-inflammatory prevention trial
- PMID: 21784351
- PMCID: PMC3149804
- DOI: 10.1016/j.jalz.2010.12.014
Extended results of the Alzheimer's disease anti-inflammatory prevention trial
Abstract
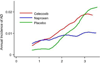
Background: Epidemiologic evidence suggests that nonsteroidal anti-inflammatory drugs (NSAIDs) delay onset of Alzheimer's dementia (AD), but randomized trials show no benefit from NSAIDs in patients with symptomatic AD. The Alzheimer's Disease Anti-inflammatory Prevention Trial (ADAPT) randomized 2,528 elderly persons to naproxen or celecoxib versus placebo for 2 years (standard deviation = 11 months) before treatments were terminated. During the treatment interval, 32 cases of AD revealed increased rates in both NSAID-assigned groups.
Methods: We continued the double-masked ADAPT protocol for 2 additional years to investigate incidence of AD (primary outcome). We then collected cerebrospinal fluid (CSF) from 117 volunteer participants to assess their ratio of CSF tau to Aβ(1-42.)
Results: Including 40 new events observed during follow-up of 2,071 randomized individuals (92% of participants at treatment cessation), there were 72 AD cases. Overall, NSAID-related harm was no longer evident, but secondary analyses showed that increased risk remained notable in the first 2.5 years of observations, especially in 54 persons enrolled with cognitive impairment--no dementia (CIND). These same analyses showed later reduction in AD incidence among asymptomatic enrollees who were given naproxen. CSF biomarker assays suggested that the latter result reflected reduced Alzheimer-type neurodegeneration.
Conclusions: These data suggest a revision of the original ADAPT hypothesis that NSAIDs reduce AD risk, as follows: NSAIDs have an adverse effect in later stages of AD pathogenesis, whereas asymptomatic individuals treated with conventional NSAIDs such as naproxen experience reduced AD incidence, but only after 2 to 3 years. Thus, treatment effects differ at various stages of disease. This hypothesis is consistent with data from both trials and epidemiological studies.
Copyright © 2011 The Alzheimer's Association. All rights reserved.
Figures








References
-
- A National Alzheimer's Strategic Plan: The Report of the Alzheimer's Study Group. [Accessed: 04 April 2009];Congressional Task Force on Alzheimer's Disease. http://www.alzstudygroup.org/Portals/0/National_Alzheimers_Strategic_Pla....
-
- Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278(5337):412–419. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

