HNK-1 glycan functions as a tumor suppressor for astrocytic tumor
- PMID: 21784847
- PMCID: PMC3173216
- DOI: 10.1074/jbc.M111.245886
HNK-1 glycan functions as a tumor suppressor for astrocytic tumor
Abstract
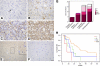
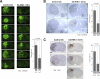
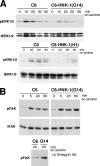
Astrocytic tumor is the most prevalent primary brain tumor. However, the role of cell surface carbohydrates in astrocytic tumor invasion is not known. In a previous study, we showed that polysialic acid facilitates astrocytic tumor invasion and thereby tumor progression. Here, we examined the role of HNK-1 glycan in astrocytic tumor invasion. A Kaplan-Meier analysis of 45 patients revealed that higher HNK-1 expression levels were positively associated with increased survival of patients. To determine the role of HNK-1 glycan, we transfected C6 glioma cells, which lack HNK-1 glycan expression, with β1,3-glucuronyltransferase-P cDNA, generating HNK-1-positive cells. When these cells were injected into the mouse brain, the resultant tumors were 60% smaller than tumors emerging from injection of the mock-transfected HNK-1-negative C6 cells. HNK-1-positive C6 cells also grew more slowly than mock-transfected C6 cells in anchorage-dependent and anchorage-independent assays. C6-HNK-1 cells migrated well after treatment of anti-β1 integrin antibody, whereas the same treatment inhibited cell migration of mock-transfected C6 cells. Similarly, α-dystroglycan containing HNK-1 glycan is different from those containing the laminin-binding glycans, supporting the above conclusion that C6-HNK-1 cells migrate independently from β1-integrin-mediated signaling. Moreover, HNK-1-positive cells exhibited attenuated activation of ERK 1/2 compared with mock-transfected C6 cells, whereas focal adhesion kinase activation was equivalent in both cell types. Overall, these results indicate that HNK-1 glycan functions as a tumor suppressor.
Figures

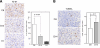



References
-
- Schachner M., Martini R. (1995) Trends. Neurosci. 18, 183–191 - PubMed
-
- Abo T., Balch C. M. (1981) J. Immunol. 127, 1024–1029 - PubMed
-
- Chou D. K., Ilyas A. A., Evans J. E., Costello C., Quarles R. H., Jungalwala F. B. (1986) J. Biol. Chem. 261, 11717–11725 - PubMed
-
- Ariga T., Kohriyama T., Freddo L., Latov N., Saito M., Kon K., Ando S., Suzuki M., Hemling M. E., Rinehart K. L., Jr. (1987) J. Biol. Chem. 262, 848–853 - PubMed
-
- Yanagisawa M., Taga T., Nakamura K., Ariga T., Yu R. K. (2005) J. Neurochem. 95, 1311–1320 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

