Adiponectin ameliorates doxorubicin-induced cardiotoxicity through Akt protein-dependent mechanism
- PMID: 21784858
- PMCID: PMC3173230
- DOI: 10.1074/jbc.M111.245985
Adiponectin ameliorates doxorubicin-induced cardiotoxicity through Akt protein-dependent mechanism
Abstract
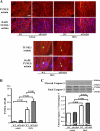
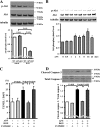
Accumulating evidence shows that obesity is associated with doxorubicin cardiac toxicity in the heart, but the molecular mechanisms that contribute to this pathological response are not understood. Adiponectin is an adipose-derived, cardioprotective factor that is down-regulated in obesity. Here, we investigated the effect of adiponectin on doxorubicin (DOX)-induced cardiotoxicity and assessed the mechanisms of this effect. A single dose of DOX was intraperitoneally injected into the abdomen of adiponectin knock-out (APN-KO) and wild-type (WT) mice. APN-KO mice had increased mortality and exacerbated contractile dysfunction of left ventricle compared with WT mice. APN-KO mice also showed increased apoptotic activity and diminished Akt signaling in the failing myocardium. Systemic delivery of adenoviral vector expressing adiponectin improved left ventricle dysfunction and myocardial apoptosis following DOX injection in WT and APN-KO mice but not in Akt1 heterozygous KO mice. In cultured rat neonatal cardiomyocytes, adiponectin stimulated Akt phosphorylation and inhibited DOX-stimulated apoptosis. Treatment with sphingosine kinase-1 inhibitor or sphingosine 1-phosphate receptor antagonist diminished adiponectin-induced Akt phosphorylation and reversed the inhibitory effects of adiponectin on myocyte apoptosis. Pretreatment with anti-calreticulin antibody reduced the binding of adiponectin to cardiac myocytes and blocked the adiponectin-stimulated increase in Akt activation and survival in cardiomyocytes. Interference of the LRP1/calreticulin co-receptor system by siRNA or blocking antibodies diminished the stimulatory actions of adiponectin on Akt activation and myocyte survival. These data show that adiponectin protects against DOX-induced cardiotoxicity by its ability to promote Akt signaling.
Figures






References
-
- Singal P. K., Iliskovic N. (1998) N. Engl. J. Med. 339, 900–905 - PubMed
-
- Arola O. J., Saraste A., Pulkki K., Kallajoki M., Parvinen M., Voipio-Pulkki L. M. (2000) Cancer Res. 60, 1789–1792 - PubMed
-
- de Azambuja E., McCaskill-Stevens W., Francis P., Quinaux E., Crown J. P., Vicente M., Giuliani R., Nordenskjöld B., Gutiérez J., Andersson M., Vila M. M., Jakesz R., Demol J., Dewar J., Santoro A., Lluch A., Olsen S., Gelber R. D., Di Leo A., Piccart-Gebhart M. (2010) Breast Cancer Res. Treat. 119, 145–153 - PubMed
-
- Mitra M. S., Donthamsetty S., White B., Mehendale H. M. (2008) Toxicol. Appl. Pharmacol. 231, 413–422 - PubMed
-
- Mitra M. S., Donthamsetty S., White B., Latendresse J. R., Mehendale H. M. (2007) Toxicol. Appl. Pharmacol. 225, 90–101 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

